Question: Please help me .. Please help me . Please please step by step answer please . I will upvote please 298 A gaseous mixture at
Please help me .. Please help me . Please please step by step answer please . I will upvote please
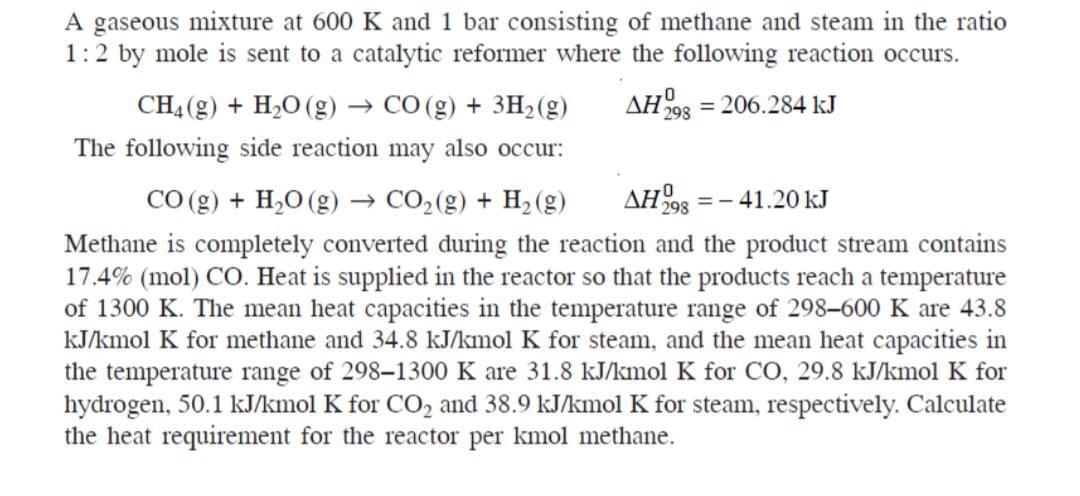
298 A gaseous mixture at 600 K and 1 bar consisting of methane and steam in the ratio 1:2 by mole is sent to a catalytic reformer where the following reaction occurs. CH4(9) + H20 (g) CO(g) + 3H2(g) AH993 = 206.284 kJ The following side reaction may also occur: CO(g) + H2O(g) + CO2(g) + H2(g) AH998 = - 41.20 kJ Methane is completely converted during the reaction and the product stream contains 17.4% (mol) CO. Heat is supplied in the reactor so that the products reach a temperature of 1300 K. The mean heat capacities in the temperature range of 298600 K are 43.8 kJ/kmol K for methane and 34.8 kJ/kmol K for steam, and the mean heat capacities in the temperature range of 2981300 K are 31.8 kJ/kmol K for CO, 29.8 kJ/kmol K for hydrogen, 50.1 kJ/kmol K for CO2 and 38.9 kJ/kmol K for steam, respectively. Calculate the heat requirement for the reactor per kmol methane
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


