Question: please help me solve #2 and #3 DATA 20. 208 Barometric pressure 767 mm Hg Trial Flavor of candy 1. Mass of candy 2. Mass
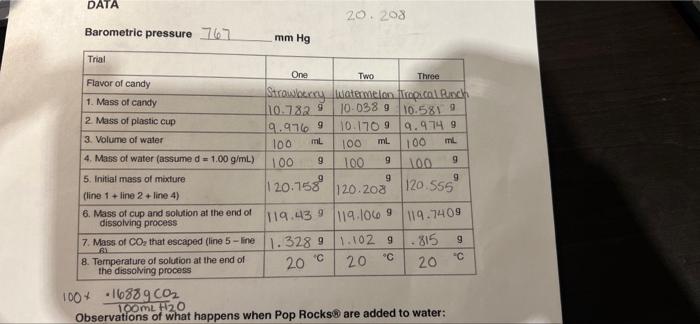
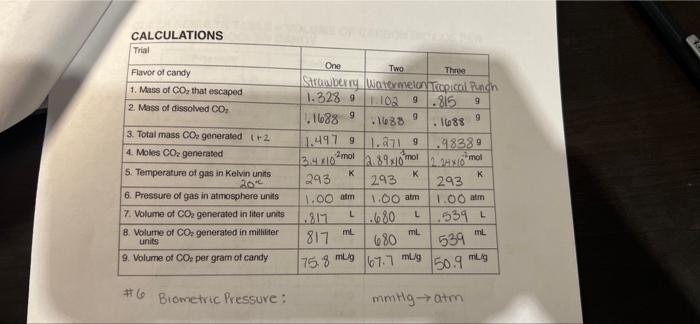

DATA 20. 208 Barometric pressure 767 mm Hg Trial Flavor of candy 1. Mass of candy 2. Mass of plastic cup 9 120.555 One Two Three Strawberry watermelon Tropical Ranch 110.7829 10-038 9 10.581 9 9.9769 10.1709 9.9749 3. Volume of water 100 mL 100 ml 100 4. Mass of water (assume d = 1.00 g/mL) 100 9 100 9 100 9 5. Initial mass of mixture 120.758 9 (line 1 line 2+ line 4) 120.203 6. Mass of cup and solution at the end of dissolving process 119.439 119.1069 119.7409 7. Mass of Co, that escaped (line 5-line 1.3289 1.102 9 BI .815 9 8. Temperature of solution at the end of 20 C " the dissolving process 100+ 16389002 TOOML 420 Observations of what happens when Pop Rocks are added to water: 200 20 CALCULATIONS Trial Flavor of candy 1. Mass of CO, that escaped 2. Mass of dissolved CO, 9 9 3. Total mass Co, generated tr2 4. Moles CO2 generated 5. Temperature of gas in Kelvin units One Two Three Strawberry Watermelon Tap.col Punch 1.328 9 1:10 9.815 1.1688 9 .1688 9 . 1688 11.497 9 1.2719 .98389 mot 3.4.40 2.0 243 K 293 293 1.00 am 1.00 atm 1.00 am .217 .680 L .539 817 ml mL mL 680 67.7 mlig 2,89x10 moi ol K K 20 6. Pressure of gas in atmosphere units L 7. Volume of Co, generated in liter units 8. Volume of CO2 generated in milliliter units 9. Volume of Co, per gram of candy 534 175.8 mug 150.9 mung #6 Biometric Pressure : mmtly atm 2 What is the volume of carbon dioxide generated in trial one corrected to STP? 3 What is the molar volume of carbon dioxide at STP based on the results of this experiment? How does this value compare to the known molar volume of 22.4 L/mol at STP
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


