Question: Q6- Choose the Correct Answer: 1. A repeatable entity of a crystal structure is known as (a) Crystal (b) Lattice (c) Unit Cell 2.
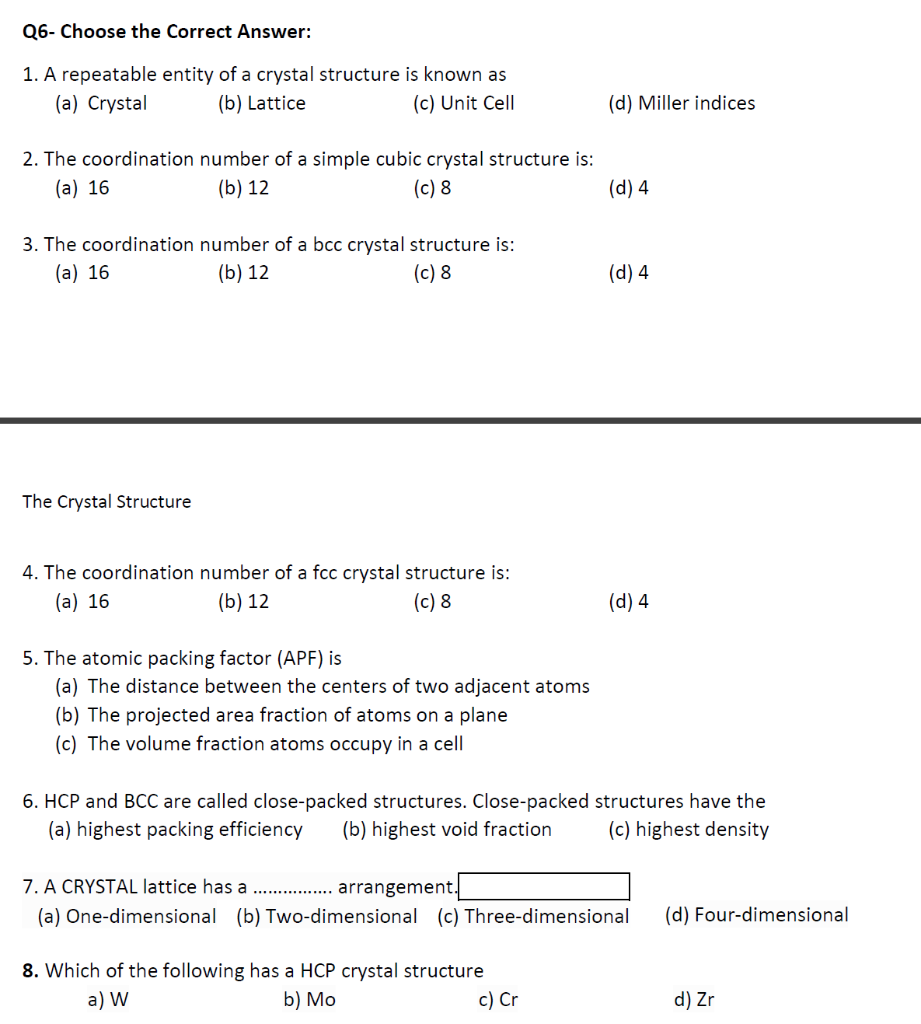
Q6- Choose the Correct Answer: 1. A repeatable entity of a crystal structure is known as (a) Crystal (b) Lattice (c) Unit Cell 2. The coordination number of a simple cubic crystal structure is: (a) 16 (b) 12 (c) 8 (d) 4 3. The coordination number of a bcc crystal structure is: (a) 16 (b) 12 (c) 8 The Crystal Structure 4. The coordination number of a fcc crystal structure is: (a) 16 (b) 12 (c) 8 5. The atomic packing factor (APF) is (a) The distance between the centers of two adjacent atoms (b) The projected area fraction of atoms on a plane (c) The volume fraction atoms occupy in a cell (d) Miller indices arrangement. (d) 4 6. HCP and BCC are called close-packed structures. Close-packed structures have the (a) highest packing efficiency (b) highest void fraction (c) highest density 8. Which of the following has a HCP crystal structure a) W b) Mo c) Cr (d) 4 7. A CRYSTAL lattice has a (a) One-dimensional (b) Two-dimensional (c) Three-dimensional (d) Four-dimensional. d) Zr
Step by Step Solution
3.39 Rating (165 Votes )
There are 3 Steps involved in it
The detailed answer for the above question is provided below Answer 1 A repeatable entity of a cryst... View full answer

Get step-by-step solutions from verified subject matter experts


