Question: please help me solve this one. Figure 1. The structures of (I-r) carbamazepine, clomipramine and omipramol; for reference, the calculated log P's (Chemaxon) are 2.77,
please help me solve this one.
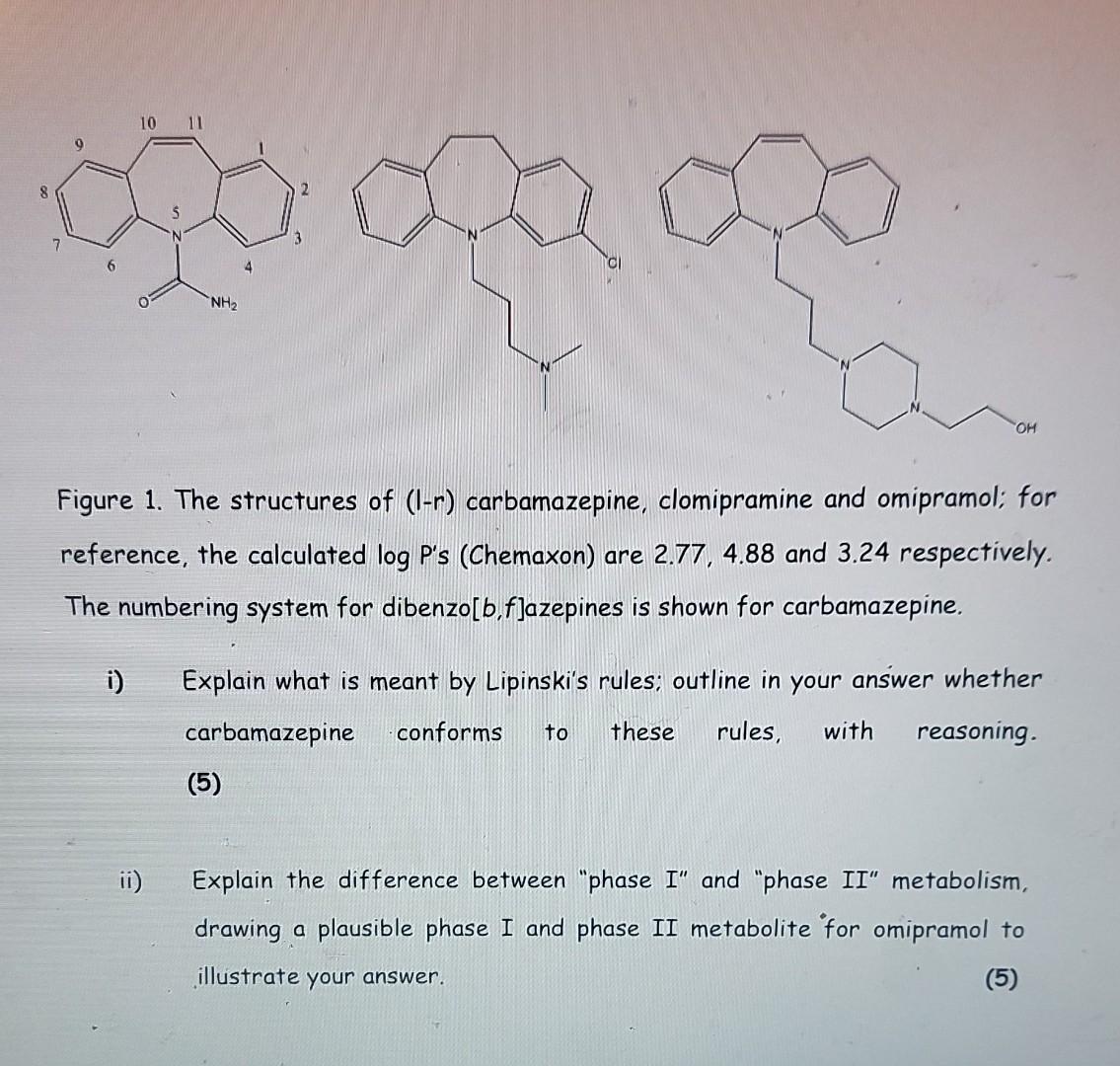

Figure 1. The structures of (I-r) carbamazepine, clomipramine and omipramol; for reference, the calculated log P's (Chemaxon) are 2.77, 4.88 and 3.24 respectively. The numbering system for dibenzo[b,f]azepines is shown for carbamazepine. i) Explain what is meant by Lipinski's rules; outline in your answer whether carbamazepine conforms to these rules, with reasoning. (5) ii) Explain the difference between "phase I" and "phase II" metabolism, drawing a plausible phase I and phase II metabolite for omipramol to illustrate your answer. (5) Part c) continued overleaf iii) Carbamazepine forms many different reactive metabolites because of CyP450-mediated oxidation, whereas clomipramine forms significantly fewer. Explain, using diagrams where appropriate, why carbamazepine can form both a 2,3-quinone derivative or a 10,11-epoxide derivative, but clomipramine will not. iv) Using a mechanism and any appropriate text, describe the role of glutathione conjugation for the detoxification of the 10,11 -epoxide of omipramol, explaining any stereochemistry in the products formed. (Use "GSH" to denote glutathione in any mechanisms in the answer.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


