Question: Please help me solve with steps, thank you! A mixture contains a volatile liquid with a pure vapor pressure of 1,000 . Torr and a
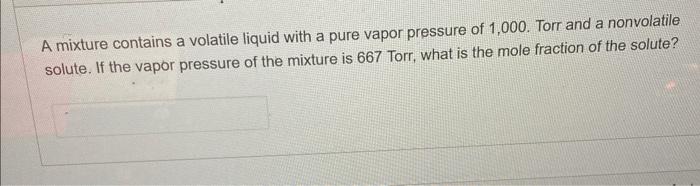
A mixture contains a volatile liquid with a pure vapor pressure of 1,000 . Torr and a nonvolatile solute. If the vapor pressure of the mixture is 667 Torr, what is the mole fraction of the solute
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


