Question: please help me. thank you! Part A If the initial reactant concentration was 0.700 M, what will The rate constant for a certain reaction is
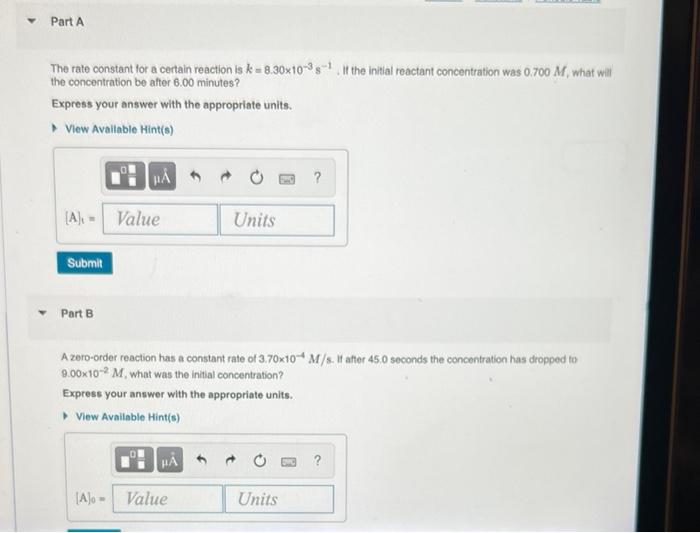
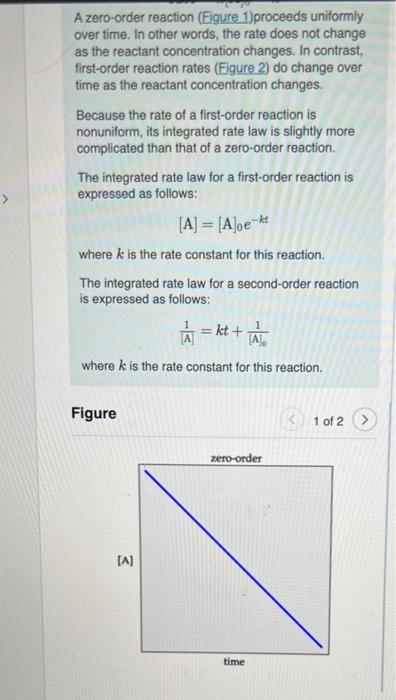
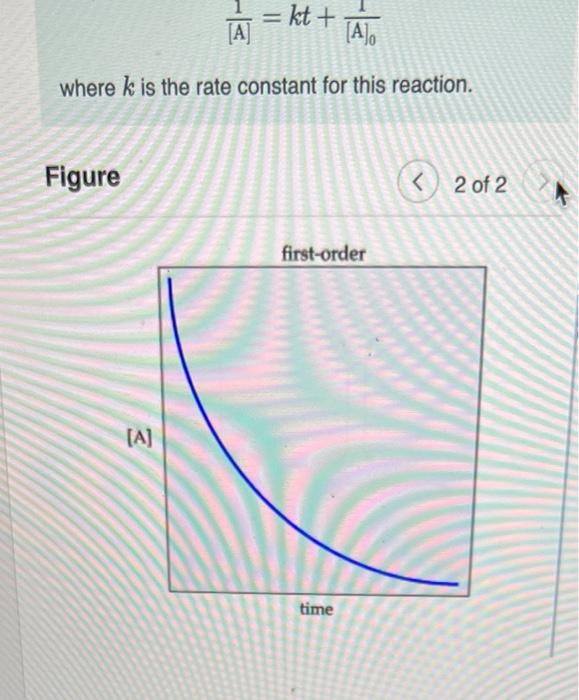
Part A If the initial reactant concentration was 0.700 M, what will The rate constant for a certain reaction is k = 8.30x10-38 the concentration be after 6.00 minutes ? Express your answer with the appropriate units. View Available Hint(s) NA ? (A): - Value Units Submit Part B A zord-order reaction has a constant rate of 3,70x10* M/s. It after 45.0 seconds the concentration has dropped to 9.00x10-2 M. what was the initial concentration? Express your answer with the appropriate units. View Available Hint(s) HA 6 ? [A]o - Value Units a A zero-order reaction (Figure 1)proceeds uniformly over time. In other words, the rate does not change as the reactant concentration changes. In contrast, first-order reaction rates (Figure 2) do change over time as the reactant concentration changes Because the rate of a first-order reaction is nonuniform, its integrated rate law is slightly more complicated than that of a zero-order reaction. The integrated rate law for a first-order reaction is expressed as follows: [A] = [Aloe-ht where k is the rate constant for this reaction. The integrated rate law for a second-order reaction is expressed as follows: = k [A where k is the rate constant for this reaction. = kt + The Figure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


