Question: please help me to solve this problem 1%; CO2=3253%) 6. The volume analysis of producer gas is : He=14% 3 CH4=2% ; CO=22. ; Con=5%;
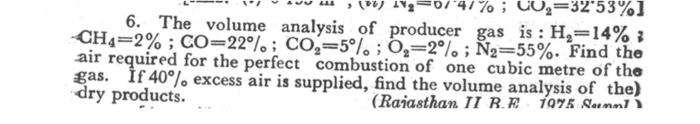
1%; CO2=3253%) 6. The volume analysis of producer gas is : He=14% 3 CH4=2% ; CO=22. ; Con=5%; 0,=2%; N2=55%. Find the air required for the perfect combustion of one cubic metre of the gas. If 40) excess air is supplied, find the volume analysis of the) dry products. (Rajasthan II RE 1975 anni
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


