Question: Please help me to solve this problem correctly and detailed. Thank you. 1. Carbon dioxide undergoes the following irreversible first-order reaction with sodium carbonate k
Please help me to solve this problem correctly and detailed. Thank you.
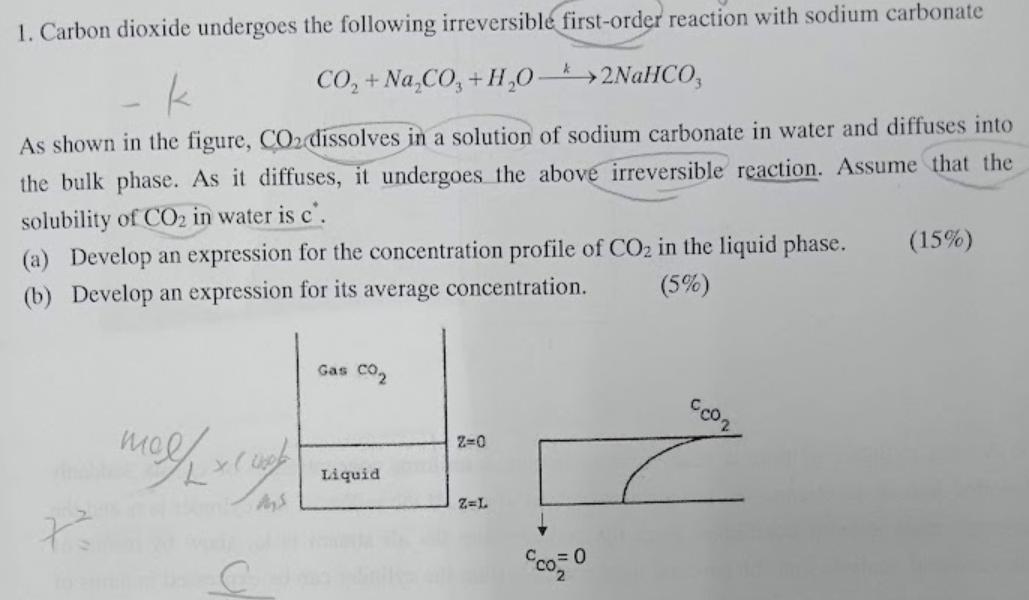
1. Carbon dioxide undergoes the following irreversible first-order reaction with sodium carbonate k - K CO, + Na_CO, +1,0->2NaHCO, As shown in the figure, CO2 dissolves in a solution of sodium carbonate in water and diffuses into the bulk phase. As it diffuses, it undergoes the above irreversible reaction. Assume that the solubility of CO2 in water is c. (a) Develop an expression for the concentration profile of CO2 in the liquid phase. (15%) (b) Develop an expression for its average concentration. (5%) Gas CO2 cor 2=0 melha cual Liquid Z=L C 9000
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


