Question: please help me to understand this assignment thanks Vapor-liquid equilibrium data for mixtures of acetone (A) and ethanol at 1 atm are given in the
please help me to understand this assignment thanks
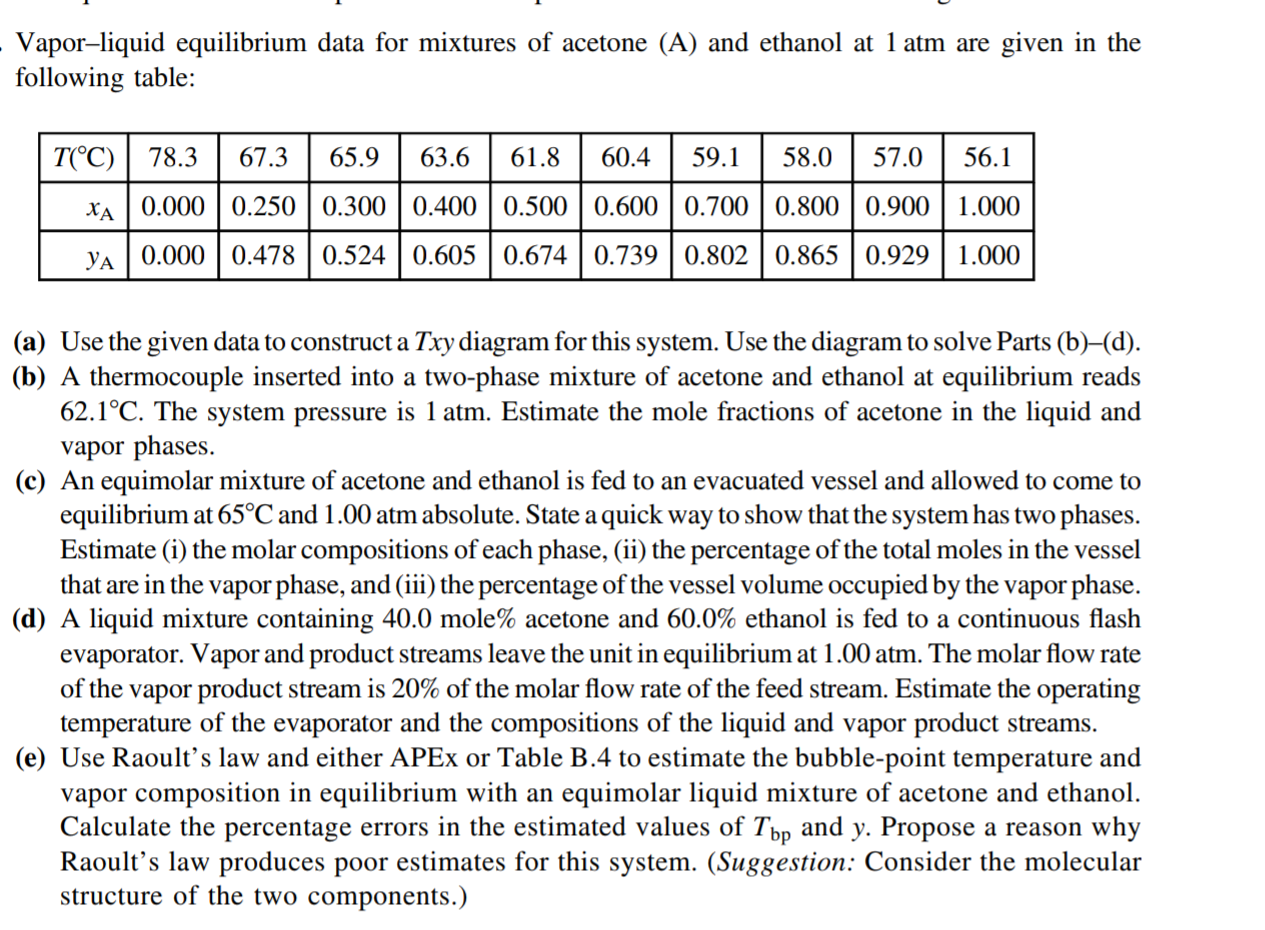
Vapor-liquid equilibrium data for mixtures of acetone (A) and ethanol at 1 atm are given in the following table: T(C) 78.3 67.3 65.9 63.6 61.8 60.4 59.1 58.0 57.0 56.1 XA | 0.000 | 0.250 | 0.300 | 0.400 0.500 0.6000.700 0.800 0.900 | 1.000 ya | 0.000 0.478 0.524 0.605 0.674 | 0.739 0.802 0.865 0.929 1.000 (a) Use the given data to construct a Txy diagram for this system. Use the diagram to solve Parts (b)(d). (b) A thermocouple inserted into a two-phase mixture of acetone and ethanol at equilibrium reads 62.1C. The system pressure is 1 atm. Estimate the mole fractions of acetone in the liquid and vapor phases. (c) An equimolar mixture of acetone and ethanol is fed to an evacuated vessel and allowed to come to equilibrium at 65C and 1.00 atm absolute. State a quick way to show that the system has two phases. Estimate (i) the molar compositions of each phase, (ii) the percentage of the total moles in the vessel that are in the vapor phase, and (iii) the percentage of the vessel volume occupied by the vapor phase. (d) A liquid mixture containing 40.0 mole% acetone and 60.0% ethanol is fed to a continuous flash evaporator. Vapor and product streams leave the unit in equilibrium at 1.00 atm. The molar flow rate of the vapor product stream is 20% of the molar flow rate of the feed stream. Estimate the operating temperature of the evaporator and the compositions of the liquid and vapor product streams. (e) Use Raoult's law and either APEx or Table B.4 to estimate the bubble-point temperature and vapor composition in equilibrium with an equimolar liquid mixture of acetone and ethanol. Calculate the percentage errors in the estimated values of Tbp and y. Propose a reason why Raoult's law produces poor estimates for this system. (Suggestion: Consider the molecular structure of the two components.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


