Question: please help me Use the Heferences to access important values I needed for this question. Sigma Bonding A o bond arises from the straight-on overlap
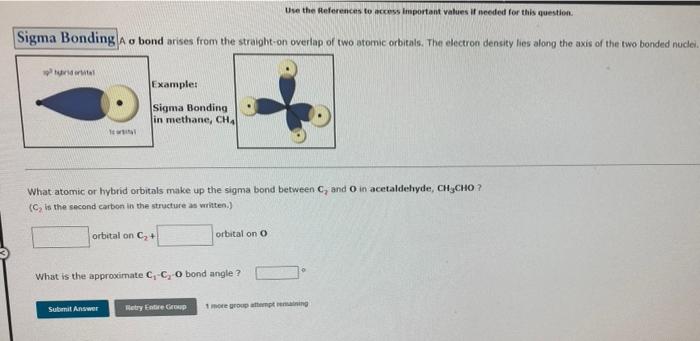
Use the Heferences to access important values I needed for this question. Sigma Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron density ties along the axis of the two bonded nuclei. Example: Sigma Bonding in methane, CHA What atomic or hybrid orbitals make up the sigma bond between C, and in acetaldehyde, CH CHO? (, is the second carbon in the structure as written.) orbital on C+ orbital on o What is the approximate C, CO bond angle? Sulit Answ Retry are up more gruppering
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


