Question: Please help me with my assignment. Data and Observations: Data Table 1: Effect of Concentration Sfock KIO, concentration is 0.02M) Data Table 2: Effect of
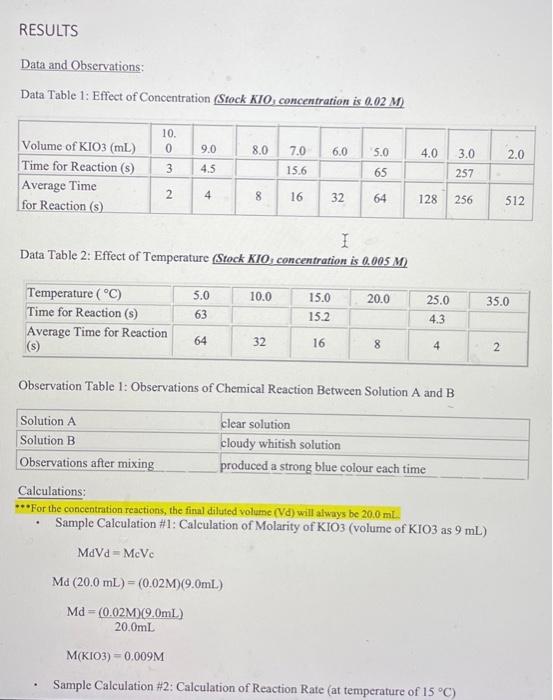
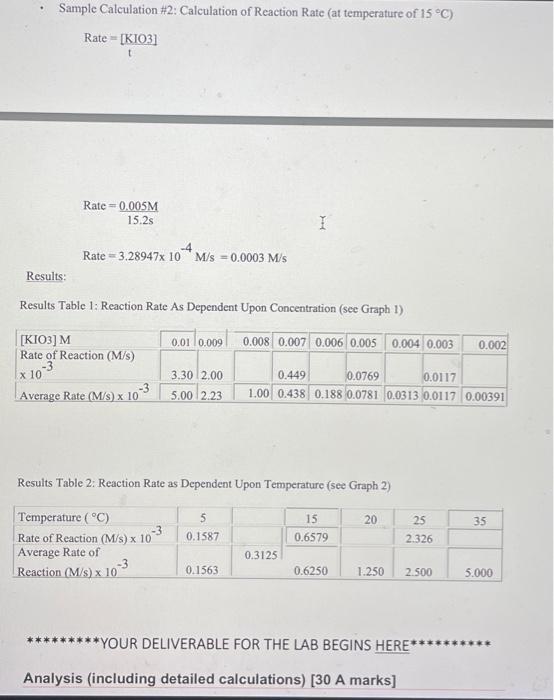
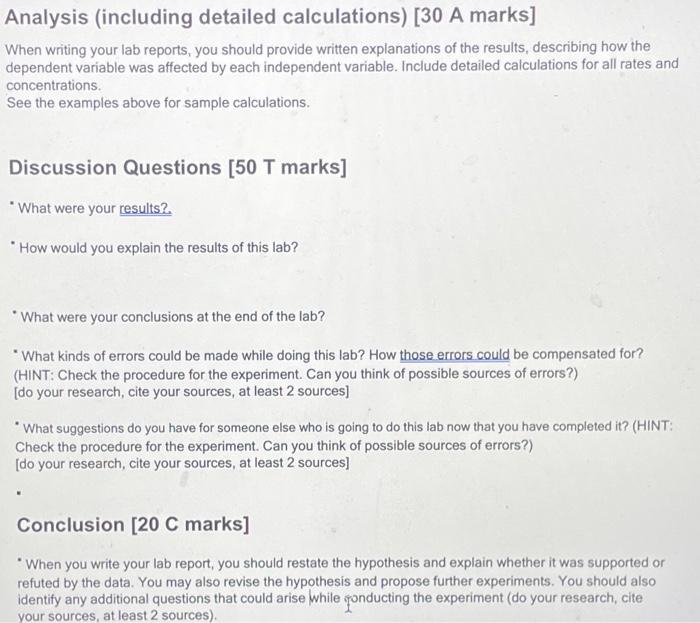
Data and Observations: Data Table 1: Effect of Concentration Sfock KIO, concentration is 0.02M) Data Table 2: Effect of Temperature Stock KIO, concentration is 0.005MA Observation Table 1: Observations of Chemical Reaction Between Solution A and B Calculations: **"For the concentration reactions, the final diluted volume (Vd) will always be 20.0mL. - Sample Calculation \#1: Calculation of Molarity of KIO3 (volume of KIO3 as 9mL ) MdVd=McVcMd(20.0mL)=(0.02M)(9.0mL)Md=20.0mL(0.02M)(9.0mL)M(KIO)=0.009M - Sample Calculation \#2: Calculation of Reaction Rate (at temperature of 15C ) - Sample Calculation \#2: Calculation of Reaction Ratc (at temperature of 15C ) Rate=t[KIO3] Rate=15.2s0.005M Rate=3.28947104M/s=0.0003M/s Results: Results Table 1: Reaction Rate As Dependent Upon Concentration (see Graph 1) Results Table 2: Reaction Rate as Dependent Upon Temperature (see Graph 2) YOUR DELIVERABLE FOR THE LAB BEGINS HERE Analysis (including detailed calculations) [30 A marks] Analysis (including detailed calculations) [ 30 A marks] When writing your lab reports, you should provide written explanations of the results, describing how the dependent variable was affected by each independent variable. Include detailed calculations for all rates and concentrations. See the examples above for sample calculations. Discussion Questions [50 T marks] "What were your results? "How would you explain the results of this lab? "What were your conclusions at the end of the lab? "What kinds of errors could be made while doing this lab? How those errors could be compensated for? (HINT: Check the procedure for the experiment. Can you think of possible sources of errors?) [do your research, cite your sources, at least 2 sources] - What suggestions do you have for someone else who is going to do this lab now that you have completed it? (HINT: Check the procedure for the experiment. Can you think of possible sources of errors?) [do your research, cite your sources, at least 2 sources] Conclusion [20 C marks] - When you write your lab report, you should restate the hypothesis and explain whether it was supported or refuted by the data. You may also revise the hypothesis and propose further experiments. You should also identify any additional questions that could arise while gonducting the experiment (do your research, cite your sources, at least 2 sources)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


