Question: Please help me with the following. 1. Activity 1: pV Diagram Cycles An ideal gas is taken around the cycle shown in this pV diagram,
Please help me with the following.
1.
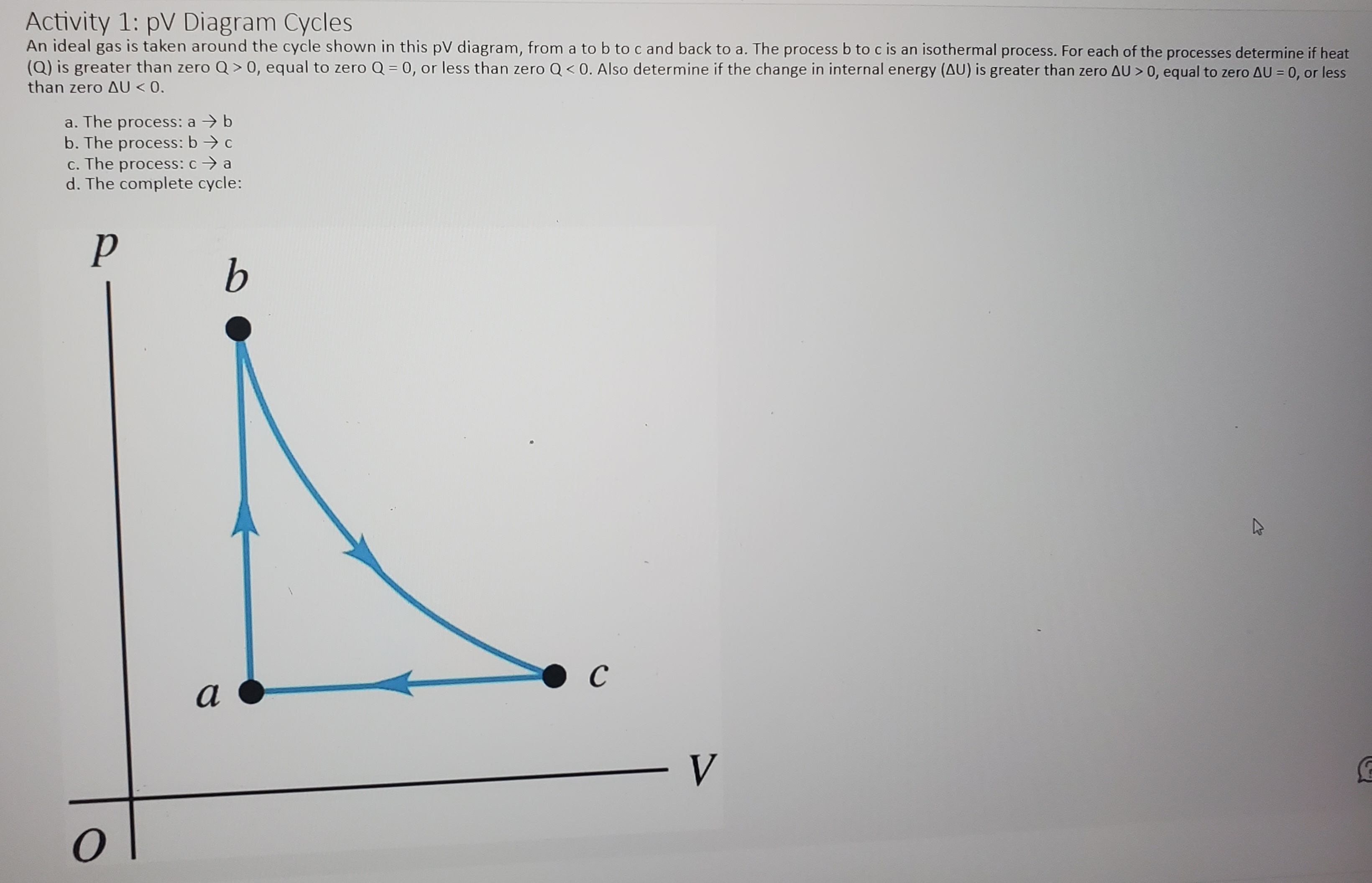
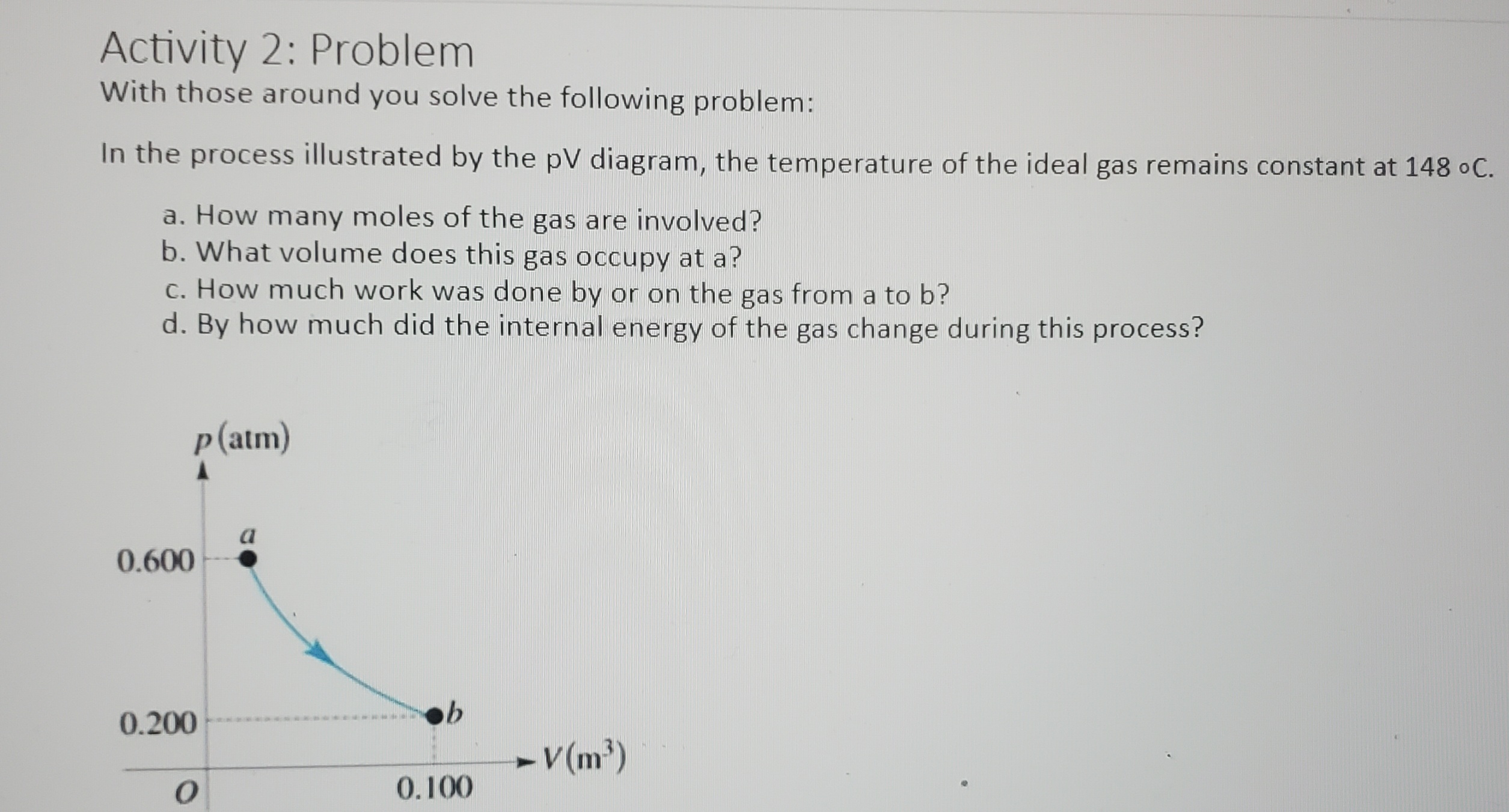
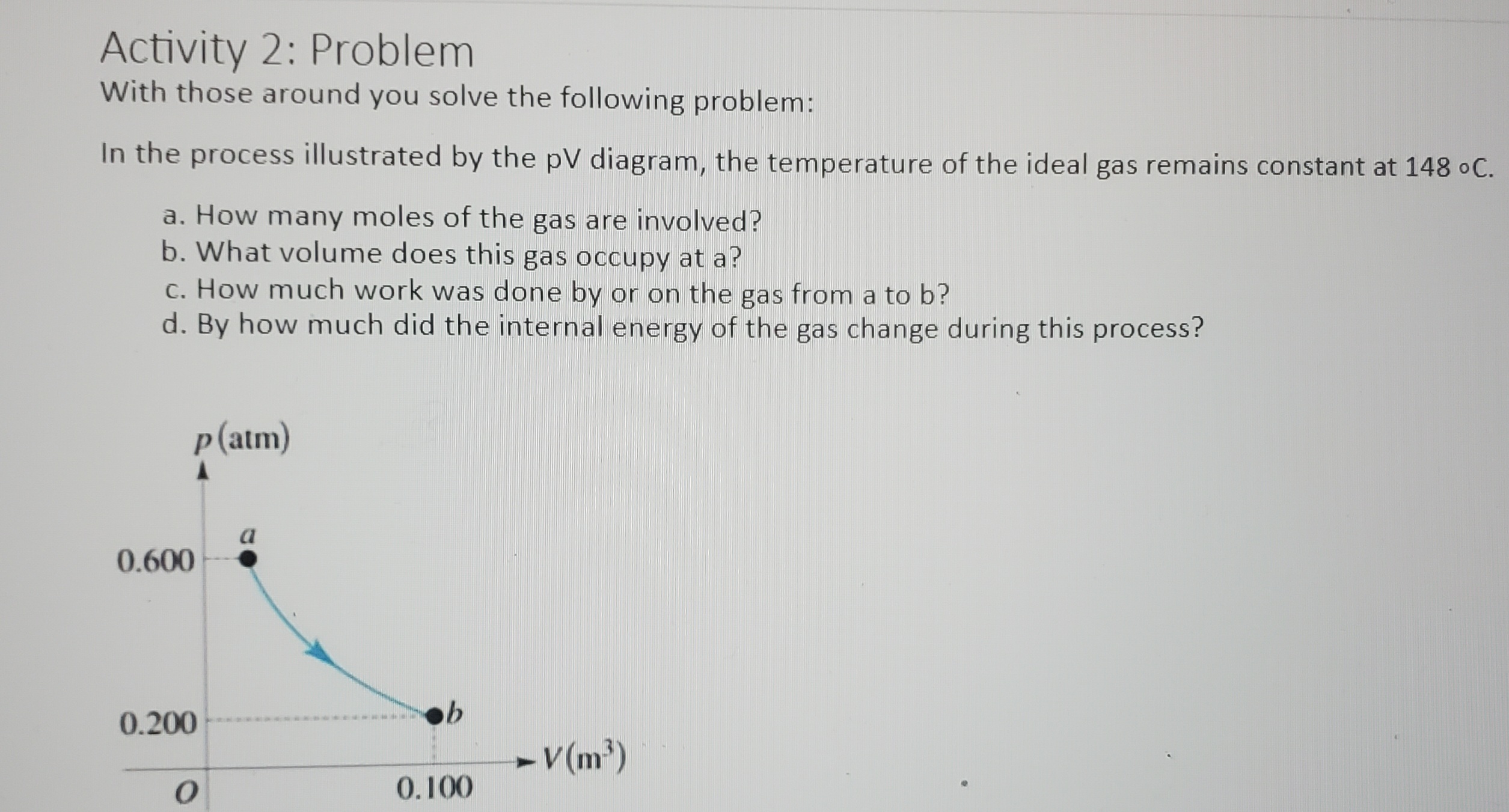
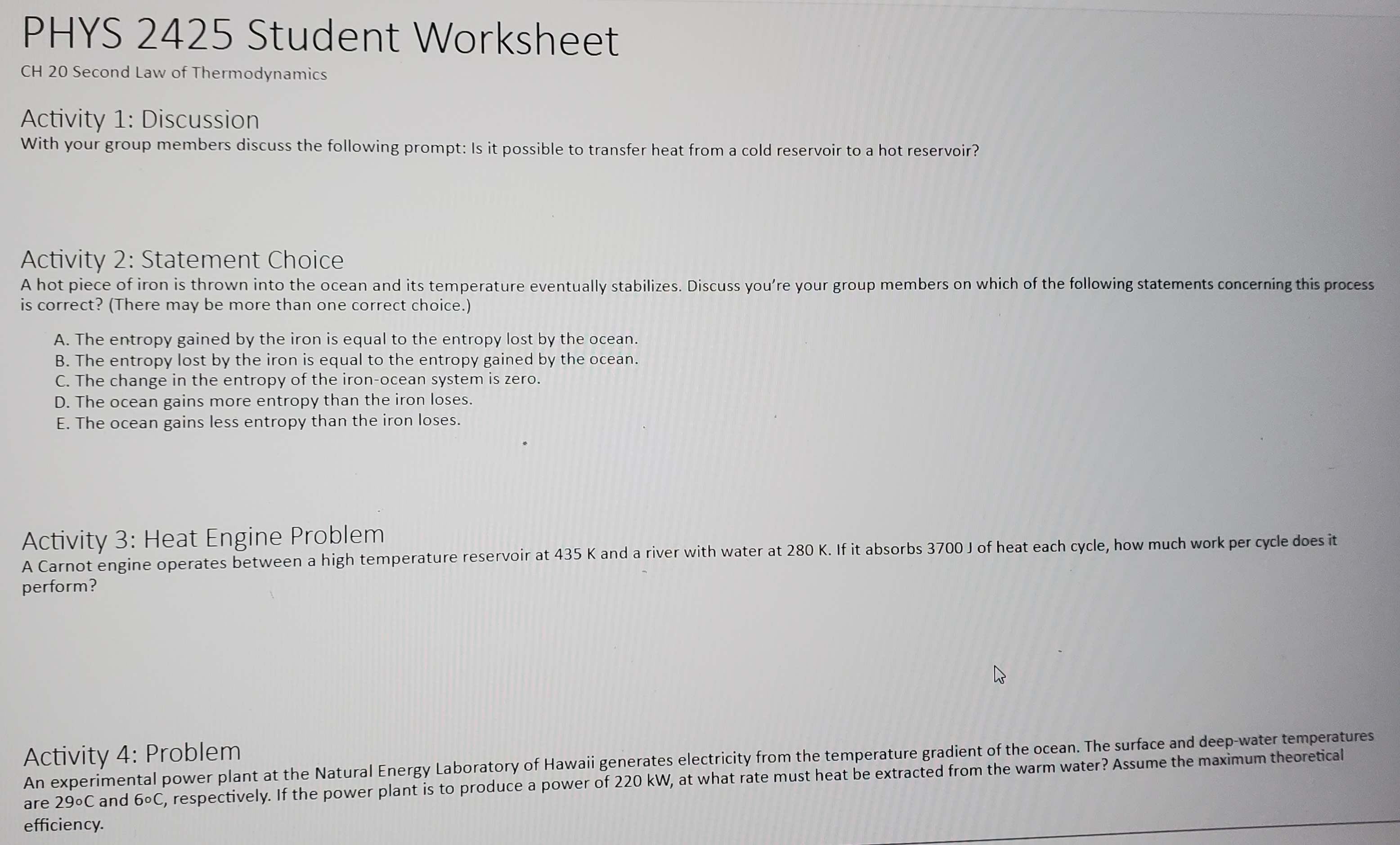
Activity 1: pV Diagram Cycles An ideal gas is taken around the cycle shown in this pV diagram, from a to b to c and back to a. The process b to c is an isothermal process. For each of the processes determine if heat (Q) is greater than zero Q > 0, equal to zero Q = 0, or less than zero Q 0, equal to zero AU = 0, or less than zero AU b b. The process: b -> c c. The process: c -> a d. The complete cycle: P b C a V OActivity 2: Problem With those around you solve the following problem: In the process illustrated by the pV diagram, the temperature of the ideal gas remains constant at 148 .C. a. How many moles of the gas are involved? b. What volume does this gas occupy at a? c. How much work was done by or on the gas from a to b? d. By how much did the internal energy of the gas change during this process? p (atm) a 0.600 0.200 - V(m3) O 0.100PHYS 2425 Student Worksheet CH 20 Second Law of Thermodynamics Activity 1: Discussion With your group members discuss the following prompt: Is it possible to transfer heat from a cold reservoir to a hot reservoir? Activity 2: Statement Choice A hot piece of iron is thrown into the ocean and its temperature eventually stabilizes. Discuss you're your group members on which of the following statements concerning this process is correct? (There may be more than one correct choice.) A. The entropy gained by the iron is equal to the entropy lost by the ocean. B. The entropy lost by the iron is equal to the entropy gained by the ocean. C. The change in the entropy of the iron-ocean system is zero. D. The ocean gains more entropy than the iron loses. E. The ocean gains less entropy than the iron loses. Activity 3: Heat Engine Problem A Carnot engine operates between a high temperature reservoir at 435 K and a river with water at 280 K. If it absorbs 3700 J of heat each cycle, how much work per cycle does it perform? Activity 4: Problem An experimental power plant at the Natural Energy Laboratory of Hawaii generates electricity from the temperature gradient of the ocean. The surface and deep-water temperatures are 290C and 60C, respectively. If the power plant is to produce a power of 220 kW, at what rate must heat be extracted from the warm water? Assume the maximum theoretical efficiency
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


