Question: Please help me with the short multiple choice questions (explanation not needed) : 1 point An ideal gas is placed in two containers. Container 1
Please help me with the short multiple choice questions (explanation not needed) :
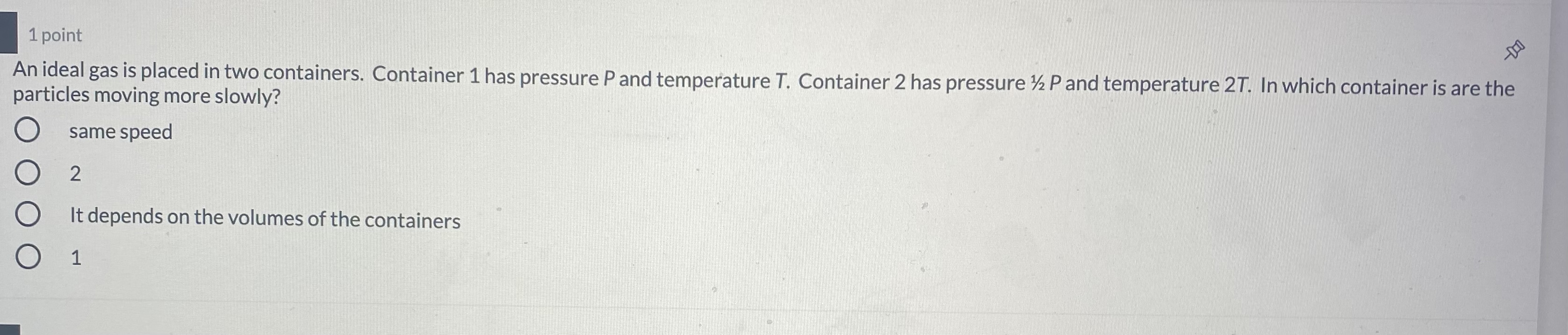




1 point An ideal gas is placed in two containers. Container 1 has pressure P and temperature T. Container 2 has pressure 12 P and temperature 27. In which container is are the particles moving more slowly? O same speed 2 O It depends on the volumes of the containers O 1An ideal gas is in a container at air pressure. Which of the following changes means the gas has increased the volume? O increasing the temperature while keeping the pressure and amount of gas constant decreasing the temperature while keeping the pressure and amount of gas constant O increasing the pressure while keeping the temperature and amount of gas constant O allowing some of the gas to leak out while keeping the temperature and pressure constant 1 point1 point An ideal gas in a container is initially at a pressure P, volume V, and temperature T. The container's pressure is tripled and the volume is doubled. What is temperature? O 6T O 2/3 T O 3/2 T O 1/6 TAn ideal gas at constant pressure has its temperature cut in half. By what factor does the rms speed of the particles in the gas change? O VZ O 1/4 4 1/2At the atomic level, what physically causes pressure to rise in a container? O As temperature goes up, the particles move faster, so they hit the walls with more force O As temperature goes up, the wall of the container gets weaker and stretches As temperature goes up, the individual particles swell up and push on the sides of the container O As temperature goes up, more particles are created
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


