Question: Please help me with this prelab. I need to use M1V1=M2V2 to calculate the volumes needed to prepare 25.00 mL of solutions 1-3. Week 1
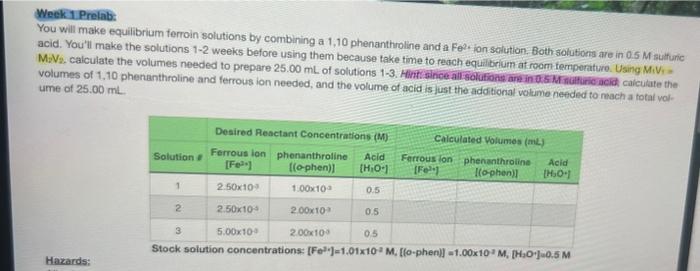
Week 1 Prelab: You will make equilibrium ferroin solutions by combining a 1,10 phenanthroline and a Fe2. fon solution. Both solutions are in 0.5M sulturic acid. You'll make the solutions 1-2 weeiss before using them because take time to reach equilibrum at room femperature. Using MiV, = M2V2. calculate the volumes needed to prepare 25.00mL of solutions 1-3. Hinti since allisolutions ars in 0 s M nualfinc acid caiculate the volumes of 1.10 phenanthroline and ferrous ion needed, and the volume of acid is usst the additional volume needed to meach a total vol- ume of 25.00mL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


