Question: please help me with this problem. I dont know how to solve it. Thank you! At equilibrium, the concentrations of reactants and products can be
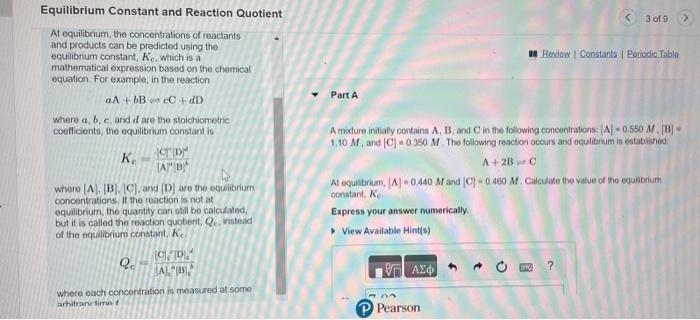
At equilibrium, the concentrations of reactants and products can be predictod using the equilibrium constant, Kc. Which is a mathermatical expression based on the chemical equation. For examplo, in the reaction aA+bB+cC+dDPartA Where a,b,c, and d are the stoichiometric coefficients, the equilibrium constant is A moture initially contains A,B, and C in the following concentrations: A=0.650M,[B]= KC=(A)4[]6C)iD)4 1,10M, and [C]=0.350M. The follawing reaction occurs and equlibrivin is estabished. whore [A],[B].[C], and [D] are the equilibrium concentrations. If the reaction is not at equilibrium, the quantity can siill be calculated, but if is called the roaction quobent, Qc instead of the equilibrium constant, Kc. Where each concentration is measured at some arhiteary time t
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


