Question: please help me with this question. step-by-step solution is highly appreciated. thank you :) 7. Use the vapor pressures of nitric acid, HNO3, given in
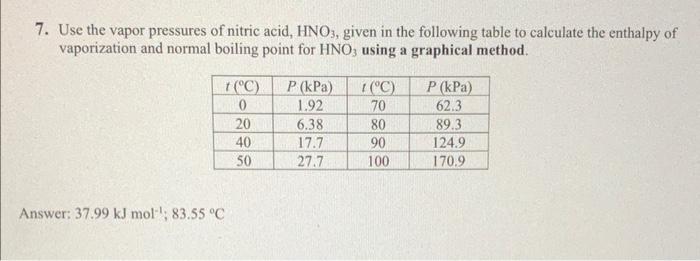
7. Use the vapor pressures of nitric acid, HNO3, given in the following table to calculate the enthalpy of vaporization and normal boiling point for HNO3 using a graphical method. Answer: 37.99kJmol1;83.55C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


