Question: please help me with those question Conformations of Ethane Construct a model of an ethane molecule. Rotate one carbon with respect to the other until
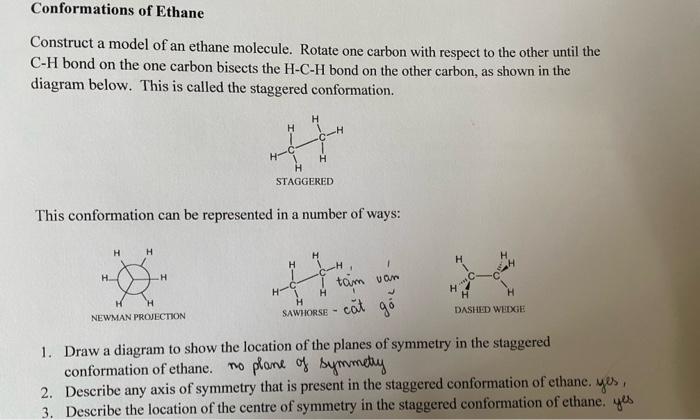
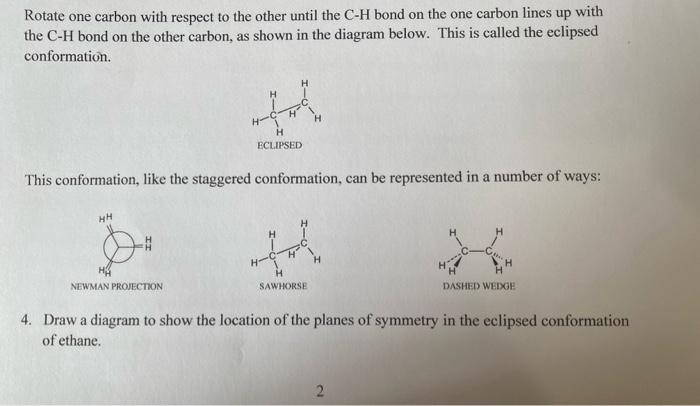

Conformations of Ethane Construct a model of an ethane molecule. Rotate one carbon with respect to the other until the CH bond on the one carbon bisects the HCH bond on the other carbon, as shown in the diagram below. This is called the staggered conformation. This conformation can be represented in a number of ways: 1. Draw a diagram to show the location of the planes of symmetry in the staggered conformation of ethane. no plane of symmetry 2. Describe any axis of symmetry that is present in the staggered conformation of ethane. yes, 3. Describe the location of the centre of symmetry in the staggered conformation of ethane. yes Rotate one carbon with respect to the other until the CH bond on the one carbon lines up with the CH bond on the other carbon, as shown in the diagram below. This is called the eclipsed conformation. This conformation, like the staggered conformation, can be represented in a number of ways: 4. Draw a diagram to show the location of the planes of symmetry in the eclipsed conformation of ethane. 5. Describe any axis of symmetry that is present in the eclipsed conformation of ethane. 6. Does the eclipsed conformation of ethane have a centre of symmetry? 7. Which of these two conformations would be the most stable? 8. What type of motion is required to interconvert these two conformers? 9. Define the term conformation. 10. The eclipsed conformation of ethane has what is called torsional strain. What is torsional strain? 11. The torsional strain in ethane has an energy of 12.5kJ/mole. Sketch a fully labelled graph of the energy of an ethane molecule against dihedral angle as one carbon is rotated with respect to the other. Start with an eclipsed conformation at 0 and go through one complete 360 revolution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


