Question: please help me without using AI tools Consider the arterial blood gas results of a patient given below: It is that the partial pressure of
please help me without using AI tools
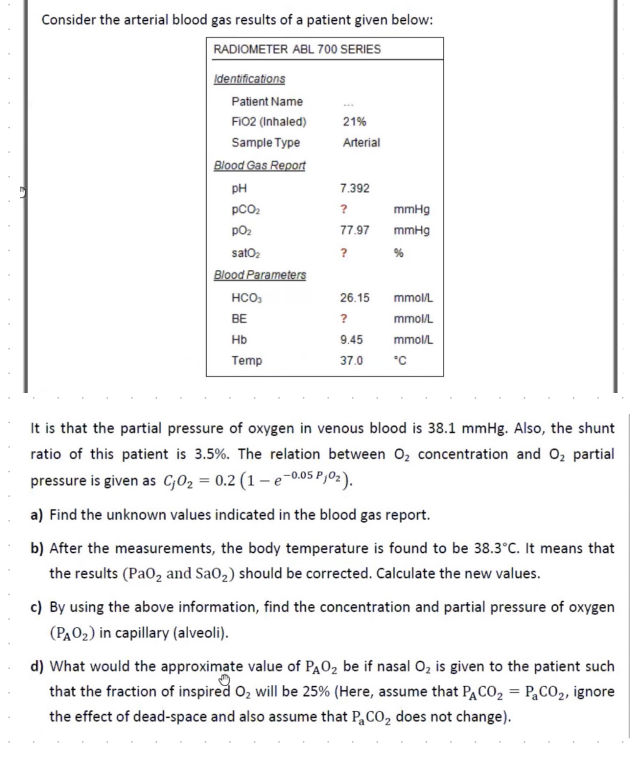
Consider the arterial blood gas results of a patient given below: It is that the partial pressure of oxygen in venous blood is 38.1mmHg. Also, the shunt ratio of this patient is 3.5%. The relation between O2 concentration and O2 partial pressure is given as CjO2=0.2(1e0.05PjO2). a) Find the unknown values indicated in the blood gas report. b) After the measurements, the body temperature is found to be 38.3C. It means that the results (PaO2 and SaO2) should be corrected. Calculate the new values. c) By using the above information, find the concentration and partial pressure of oxygen (PAO2) in capillary (alveoli). d) What would the approximate value of PAO2 be if nasal O2 is given to the patient such that the fraction of inspired O2 will be 25% (Here, assume that PACO2=PaaCO2, ignore the effect of dead-space and also assume that PaCO2 does not change)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


