Question: Please help // Need ASAP Solids and liquids will generally have increased solubility when heated, but for gases, the opposite is true. As temperature increases,
Please help // Need ASAP
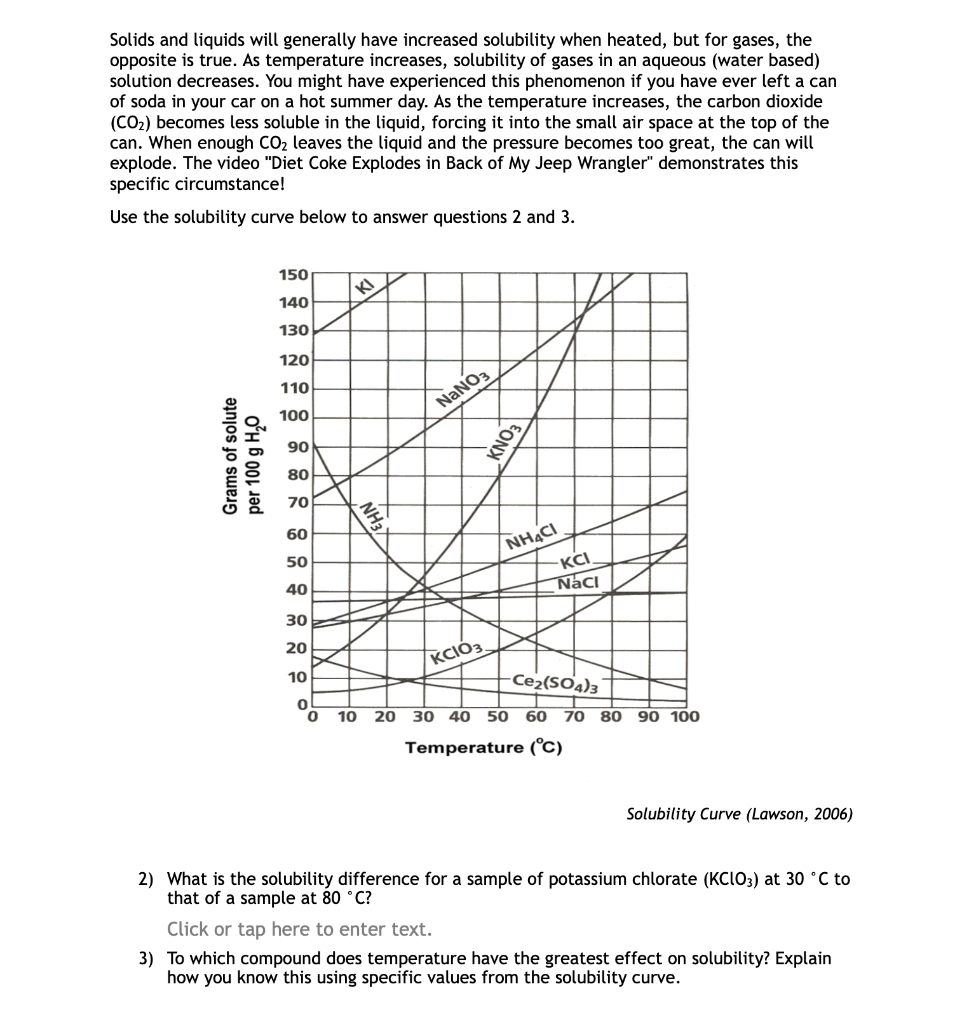
Solids and liquids will generally have increased solubility when heated, but for gases, the opposite is true. As temperature increases, solubility of gases in an aqueous (water based) solution decreases. You might have experienced this phenomenon if you have ever left a can of soda in your car on a hot summer day. As the temperature increases, the carbon dioxide (CO2) becomes less soluble in the liquid, forcing it into the small air space at the top of the can. When enough CO2 leaves the liquid and the pressure becomes too great, the can will explode. The video "Diet Coke Explodes in Back of My Jeep Wrangler" demonstrates this specific circumstance! Use the solubility curve below to answer questions 2 and 3. Solubility Curve (Lawson, 2006) 2) What is the solubility difference for a sample of potassium chlorate (KClO3) at 30C to that of a sample at 80C ? Click or tap here to enter text. 3) To which compound does temperature have the greatest effect on solubility? Explain how you know this using specific values from the solubility curve
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


