Question: please help! need help solving this is a practice review. How many moles are present in a sample if it consists of 5.611022 particles? (NA
please help! need help solving this is a practice review.
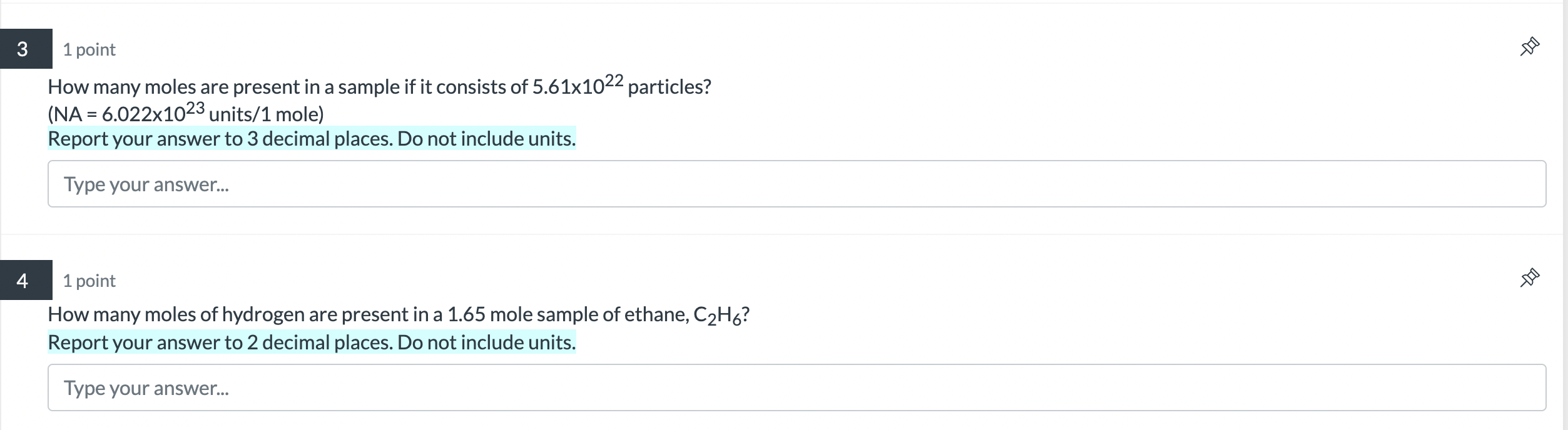
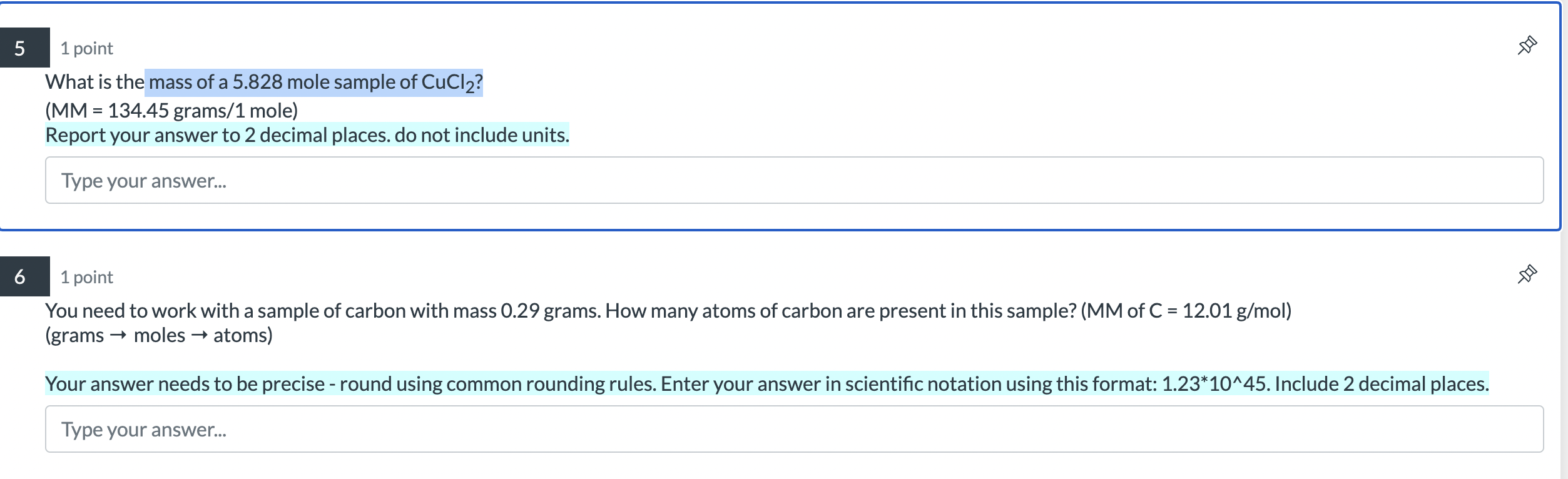
How many moles are present in a sample if it consists of 5.611022 particles? (NA =6.0221023 units /1 mole) Report your answer to 3 decimal places. Do not include units. Type your answer... 1 point How many moles of hydrogen are present in a 1.65 mole sample of ethane, C2H6 ? Report your answer to 2 decimal places. Do not include units. Type your answer... 1 point What is the mass of a 5.828 mole sample of CuCl2 ? (MM = 134.45 grams/1 mole) Report your answer to 2 decimal places. do not include units. Type your answer... 1 point You need to work with a sample of carbon with mass 0.29 grams. How many atoms of carbon are present in this sample? (MM of C=12.01g/mol) (grams moles atoms) Your answer needs to be precise - round using common rounding rules. Enter your answer in scientific notation using this format: 1.231045. Include 2 decimal places. Type your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


