Question: please help. ochem2. not choices 4 or 6 Explain the vast difference in pK2 values for the following two apparently similar compounds, is the stronger
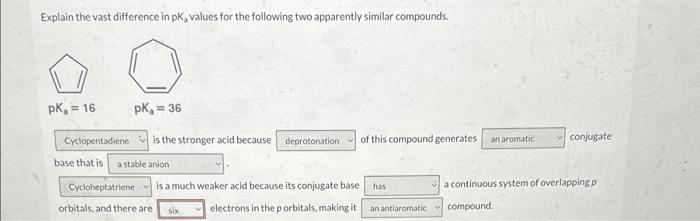
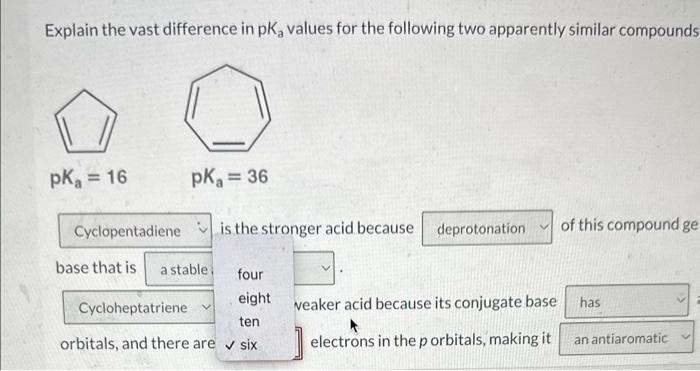
Explain the vast difference in pK2 values for the following two apparently similar compounds, is the stronger acid because of this compound generates. conjugate base that is is a much weaker acid because its conjugate base a continuous system of overlapping orbitals, and there are electrons in the p orbitals, making it compound. Explain the vast difference in pKa values for the following two apparently similar compounds is the stronger acid because of this compound ge base that is four eight veaker acid because its conjugate base ten orbitals, and there are six ] electrons in the p orbitals, making it
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


