Question: please help on number 2 a,b, and c. 2. Using pKa values, you can predict the position of equilibrium for any acid-base reaction. Predict which
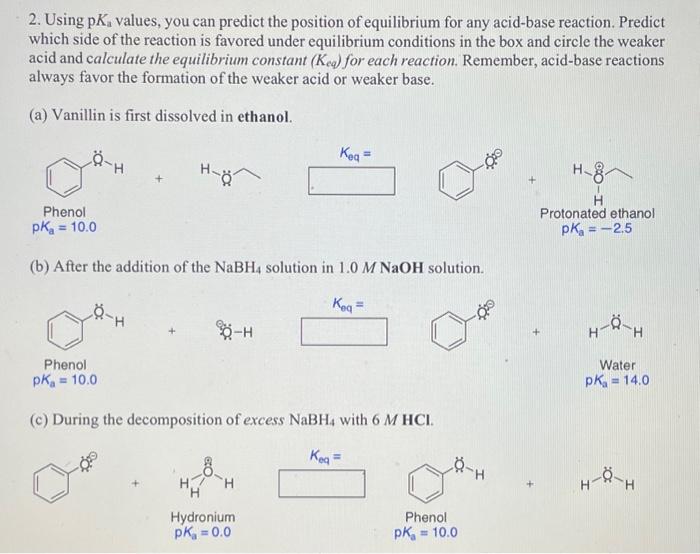
2. Using pKa values, you can predict the position of equilibrium for any acid-base reaction. Predict which side of the reaction is favored under equilibrium conditions in the box and circle the weaker acid and calculate the equilibrium constant (Keq) for each reaction. Remember, acid-base reactions always favor the formation of the weaker acid or weaker base. (a) Vanillin is first dissolved in ethanol. Keq= Phenol pKa=10.0 Protonated ethanol pKa=2.5 (b) After the addition of the NaBH4 solution in 1.0MNaOH solution. +88H Keq= Phenol Water pKa=10.0 pKa=14.0 (c) During the decomposition of excess NaBH4 with 6MHCl. Keq= Hydronium Phenol pKa=0.0 pKa=10.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


