Question: Please help solve this question 6. Draw a reaction mechanism using the curved-arrow formalism (include lone pair electrons) for the conversion of methyl salicylate into
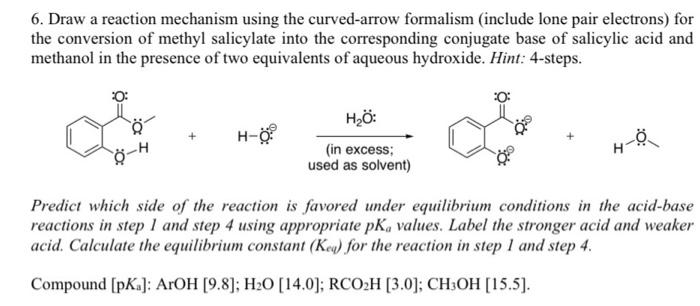
6. Draw a reaction mechanism using the curved-arrow formalism (include lone pair electrons) for the conversion of methyl salicylate into the corresponding conjugate base of salicylic acid and methanol in the presence of two equivalents of aqueous hydroxide. Hint: 4-steps. +H00 Predict which side of the reaction is favored under equilibrium conditions in the acid-base reactions in step 1 and step 4 using appropriate pKa values. Label the stronger acid and weaker acid. Calculate the equilibrium constant (Kequ ) for the reaction in step 1 and step 4. Compound [pKa]:ArOH[9.8];H2O[14.0];RCO2H[3.0];CH3OH[15.5]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


