Question: please help out soon Exercise 1. Answer all the following questions (3 marks): 1. What is the change of entropy (AS) for an irreversible process
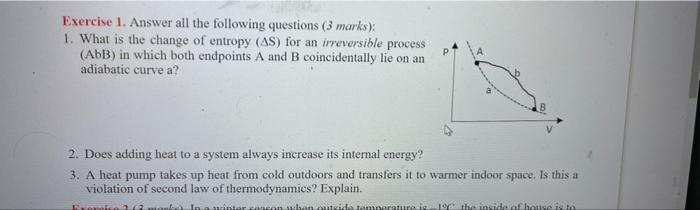
Exercise 1. Answer all the following questions (3 marks): 1. What is the change of entropy (AS) for an irreversible process (ADB) in which both endpoints A and B coincidentally lie on an adiabatic curve a? P 2. Does adding heat to a system always increase its internal energy? 3. A heat pump takes up heat from cold outdoors and transfers it to warmer indoor space. Is this a violation of second law of thermodynamics? Explain. Resimtareasonabutaidemarare the inside af house
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


