Question: Please Help! Please show full steps. Include ICE table if applicable. Thank you! The common amino acids are polyprotic acids. All amino acids contain an
Please Help! Please show full steps. Include ICE table if applicable. Thank you!
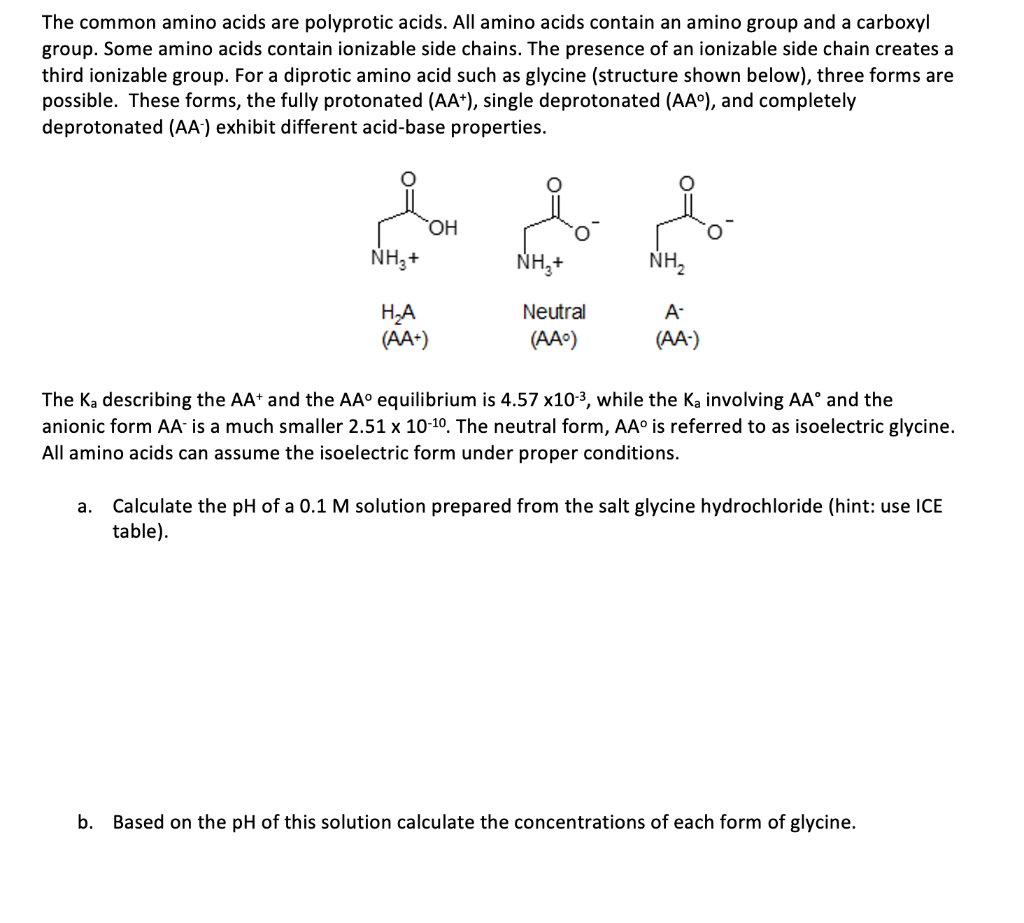

The common amino acids are polyprotic acids. All amino acids contain an amino group and a carboxyl group. Some amino acids contain ionizable side chains. The presence of an ionizable side chain creates a third ionizable group. For a diprotic amino acid such as glycine (structure shown below), three forms are possible. These forms, the fully protonated (AA+), single deprotonated (AA), and completely deprotonated (AA-) exhibit different acid-base properties. NH+ NH,+ ofra (AA+) Neutral (AA) (AA) The Ka describing the AA+ and the AA equilibrium is 4.57 x10-3, while the Ka involving AA and the anionic form AA is a much smaller 2.51 x 10-10. The neutral form, AA is referred to as isoelectric glycine. All amino acids can assume the isoelectric form under proper conditions. a. Calculate the pH of a 0.1 M solution prepared from the salt glycine hydrochloride (hint: use ICE table). b. Based on the pH of this solution calculate the concentrations of each form of glycine. C. A 20 mL solution of 0.1 M glycine hydrochloride has been prepared. How many moles of the weak acid and conjugate base forms of glycine are present after the addition of 620 ul of 1.0 M NaOH? What is the pH of the resulting solution? d. What is the pH of the solution from (C) after the addition of another 1000 uL of 1.0 M NaOH? Which forms of glycine are present? e. What is the pH of the solution from (D) after the addition of another 1000 ul of 1.0 M NaOH? Which forms of glycine are present? f. What is the pH of the solution from (E) after the addition of sufficient NaOH to convert all the glycine to the AA form
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


