Question: please help solve. please show all work so I can inderstand how to work out problem 3) Examine the phase diagram for the substance Phonium
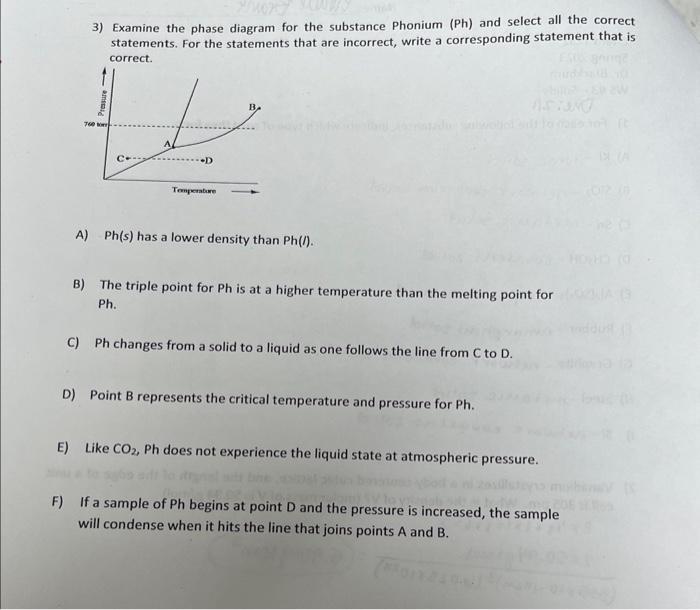
3) Examine the phase diagram for the substance Phonium ( Ph) and select all the correct statements. For the statements that are incorrect, write a corresponding statement that is correct. A) Ph(s) has a lower density than Ph(l). B) The triple point for Ph is at a higher temperature than the melting point for Ph. C) Ph changes from a solid to a liquid as one follows the line from C to D. D) Point B represents the critical temperature and pressure for Ph. E) Like CO2,Ph does not experience the liquid state at atmospheric pressure. ) If a sample of Ph begins at point D and the pressure is increased, the sample will condense when it hits the line that joins points A and B
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


