Question: please help solve these 3 questions. please include steps taken so i can understand how to work them out. Pre-Lab Questions 1. The following data
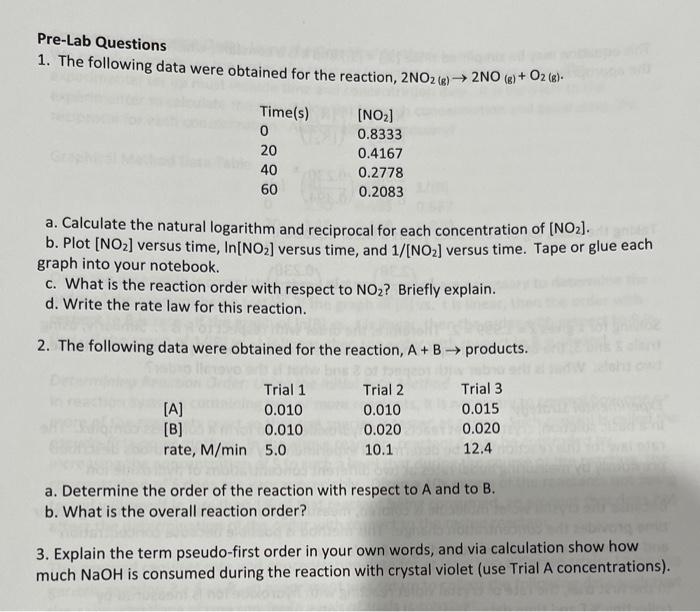
Pre-Lab Questions 1. The following data were obtained for the reaction, 2NO2(g)2NO(g)+O2(g). a. Calculate the natural logarithm and reciprocal for each concentration of [NO2]. b. Plot [NO2] versus time, ln[NO2] versus time, and 1/[NO2] versus time. Tape or glue each graph into your notebook. c. What is the reaction order with respect to NO2 ? Briefly explain. d. Write the rate law for this reaction. 2. The following data were obtained for the reaction, A+B products. a. Determine the order of the reaction with respect to A and to B. b. What is the overall reaction order? 3. Explain the term pseudo-first order in your own words, and via calculation show how much NaOH is consumed during the reaction with crystal violet (use Trial A concentrations)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


