Question: please help solve with steps shown Concentrations for Mixture D: acetone: 0.800 H+: 0.200 I2: 0.0005 PROBLEM SET - Use provi Fill Table 4 below.
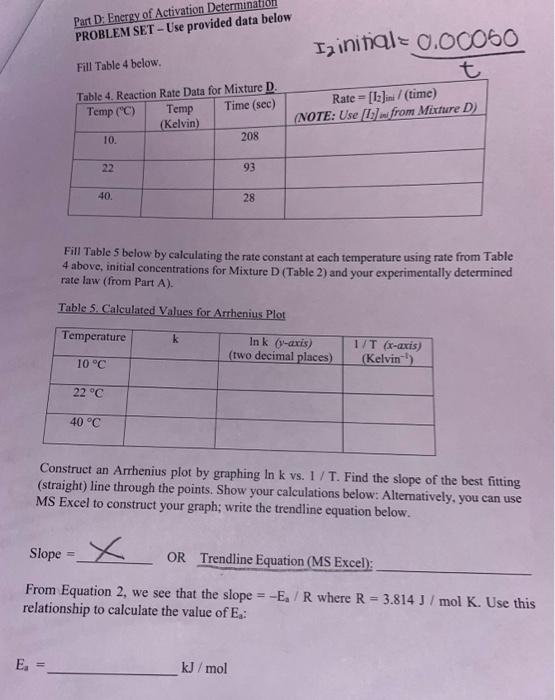
PROBLEM SET - Use provi Fill Table 4 below. I2 initial =t0.00050 Fill Table 5 below by calculating the rate constant at each temperature using rate from Table 4 above, initial concentrations for Mixture D (Table 2) and your experimentally determined rate law (from Part A). Table 5. Calculated Values for Arrhenius Plot Construet an Arrhenius plot by graphing ln kvs.1 / T. Find the slope of the best fitting (straight) line through the points. Show your calculations below: Altematively, you can use MS Excel to construct your graph; write the trendline equation below. Slope = From Equation 2, we see that the slope =Ea/R where R=3.814J/molK. Use this relationship to calculate the value of Ea : PROBLEM SET - Use provi Fill Table 4 below. I2 initial =t0.00050 Fill Table 5 below by calculating the rate constant at each temperature using rate from Table 4 above, initial concentrations for Mixture D (Table 2) and your experimentally determined rate law (from Part A). Table 5. Calculated Values for Arrhenius Plot Construet an Arrhenius plot by graphing ln kvs.1 / T. Find the slope of the best fitting (straight) line through the points. Show your calculations below: Altematively, you can use MS Excel to construct your graph; write the trendline equation below. Slope = From Equation 2, we see that the slope =Ea/R where R=3.814J/molK. Use this relationship to calculate the value of Ea
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


