Question: please help !! Some ions are sketched below. Rank them in increasing order of size of hydration enthalpy Hhyd. That is, select 1 next to
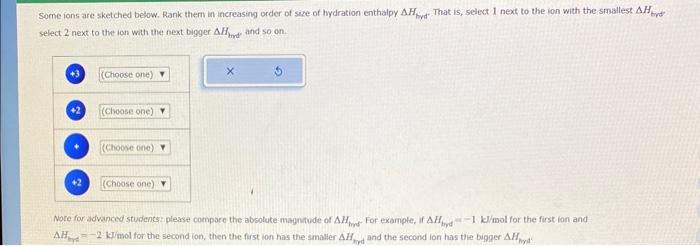
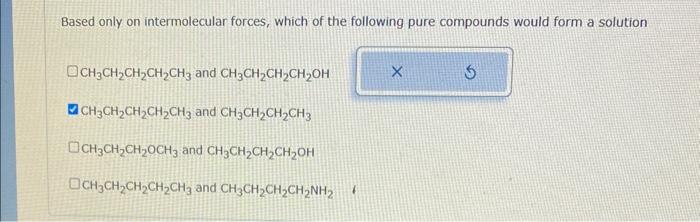
Some ions are sketched below. Rank them in increasing order of size of hydration enthalpy Hhyd. That is, select 1 next to the ion with the smallest Htyd. select 2 next to the ion with the next bigger Hhnd and so on. Noce for advanced students: please compare the absolute magnitude of Hhyd For example, if Hhyd=1k/mol for the first ion and A Hhyd=2kJ imol for the second ion, then the first ion has the smaller Hhrd and the second ion has the bigger Hhyd. Based only on intermolecular forces, which of the following pure compounds would form a solution CH3CH2CH2CH2CH3 and CH3CH2CH2CH2OH CH3CH2CH2CH2CH3 and CH3CH2CH2CH3 CH3CH2CH2OCH3 and CH3CH2CH2CH2OH CH3CH2CH2CH2CH3 and CH3CH2CH2CH2NH2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


