Question: PLEASE HELP SOON Question 10 0 / 4 pts A Hydrogen atom has an electron in n = 4. It absorbs a photon and the
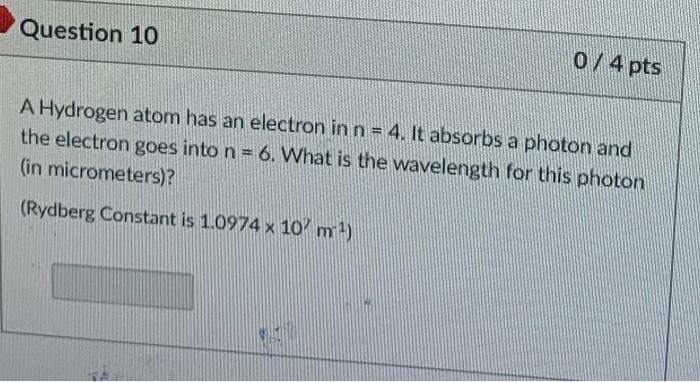
Question 10 0 / 4 pts A Hydrogen atom has an electron in n = 4. It absorbs a photon and the electron goes into n = 6. What is the wavelength for this photon (in micrometers)? (Rydberg Constant is 1.0974 x 10 m )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


