Question: Please help!! than you! what is the net ionic equation (if any): Mix aqueous potassium chromate with aqueous barium nitrate: CrO42(aq)+Ba+2(aq)BaCrO4(s)2K+(aq)+CrO42(aq)+Ba+2(aq)+2NO3(aq)BaCrO4(s)+2KNO3(aq) No net ionic equation
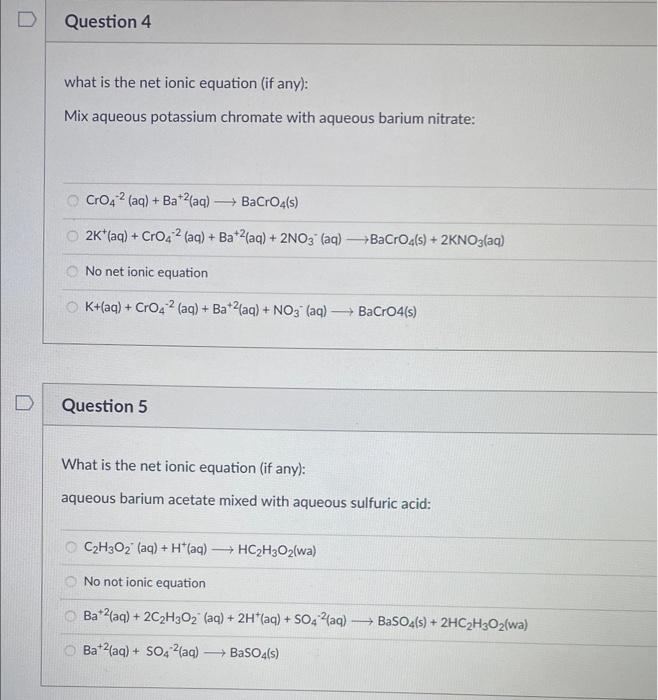
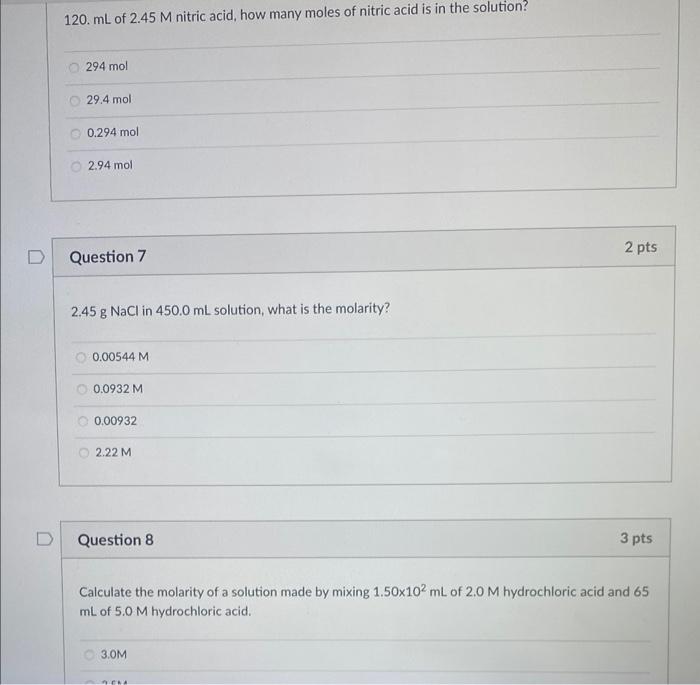

what is the net ionic equation (if any): Mix aqueous potassium chromate with aqueous barium nitrate: CrO42(aq)+Ba+2(aq)BaCrO4(s)2K+(aq)+CrO42(aq)+Ba+2(aq)+2NO3(aq)BaCrO4(s)+2KNO3(aq) No net ionic equation K+(aq)+CrO42(aq)+Ba+2(aq)+NO3(aq)BaCrO(s) Question 5 What is the net ionic equation (if any): aqueous barium acetate mixed with aqueous sulfuric acid: C2H3O2(aq)+H+(aq)HC2H3O2(wa) No not ionic equation Ba+2(aq)+2C2H3O2(aq)+2H+(aq)+SO42(aq)BaSO4(s)+2HC2H3O2(wa)Ba+2(aq)+SO42(aq)BaSO4(s) 120. mL of 2.45M nitric acid, how many moles of nitric acid is in the solution? 294mol 29.4mol 0.294mol 2.94mol Question 7 2pts 2.45gNaCl in 450.0mL solution, what is the molarity? 0.00544M 0.0932M 0.00932 2.22M Question 8 3pts Calculate the molarity of a solution made by mixing 1.50102mL of 2.0M hydrochloric acid and 65 mL of 5.0M hydrochloric acid. 3.0M How many mL of 35.0%(g/g)HCl is needed to be diluted to 1.00L to make a 5.50MHCl solution? The density of 35.0%HCl is 1.18g/mL 468mL 474mL 447mL 486mL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


