Question: please help, thank you! A mixture initially contains A,B, and C in the following concontrations: [A]=0.550M,[B]=1.20,M, and [C]=0.400,M. The following reaction occurs and equilibrium is
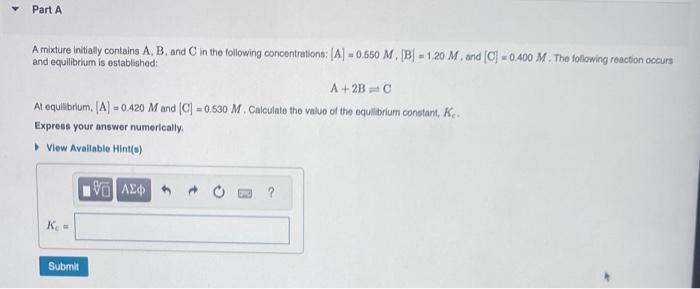
A mixture initially contains A,B, and C in the following concontrations: [A]=0.550M,[B]=1.20,M, and [C]=0.400,M. The following reaction occurs and equilibrium is established: A+2BC At equilibrium, [A]=0.420M and [C]=0.530M, Calculate the valus of the equllbrium constant, Ke. Express your answer numerically
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


