Question: PLEASE HELP Under what conditions will a gas start to behave in a non-ideal fashion? (Check all that apply) O A. When the pressure is

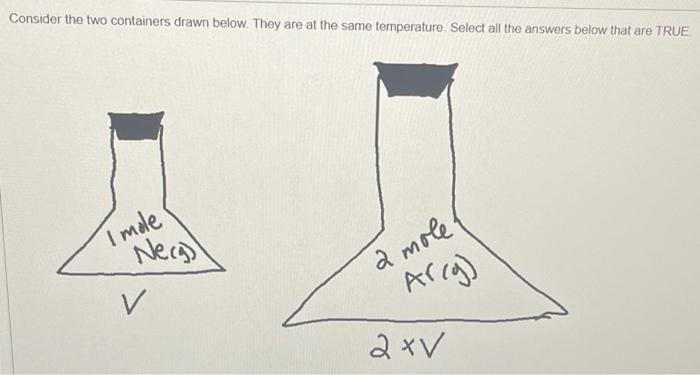

Under what conditions will a gas start to behave in a non-ideal fashion? (Check all that apply) O A. When the pressure is altered due to attractions between neighboring gas particles B. When the volume of the container becomes small enough such that the total volume of the gas particles themselves become relevant C. When the temperature is lowered D. When the volume increases with a corresponding decrease in pressure Consider the two containers drawn below. They are at the same temperature Select all the answers below that are TRUE Imole Necg) 2 mole Arcg) v A. On average, the average velocity of the argon atoms in the large container is higher than the average velocity of the neon atoms in the small container B. On average, the average velocity of the argon atoms in the large container is lower than the average velocity of the neon atoms in the small container C. The average kinetic energy of the Neon gas in the small container is the same as the average kinetic energy of the argon in the large container OD.On average, the average velocity of the argon atoms in the large container is the same as the average velocity of the neon atoms in the small container D E. The pressure in the large container is lower than the pressure in the small container O F. The Pressure in both containers are the same
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


