Question: Please Help Use the data given in the table to calculate the value of Grin at 25C for the reaction described by the equation A+BC
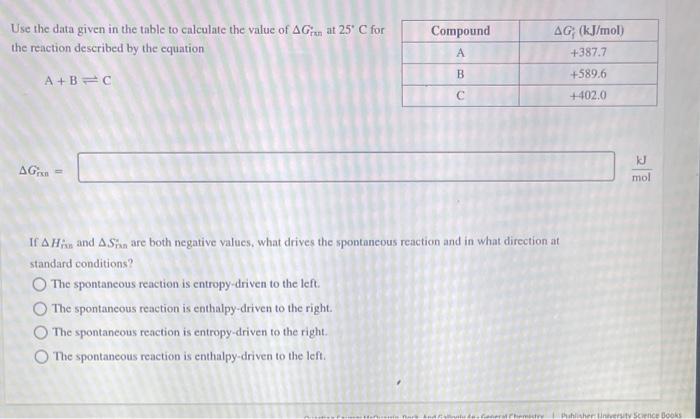
Use the data given in the table to calculate the value of Grin at 25C for the reaction described by the equation A+BC Grnn= If Hme and Sma are both negative values, what drives the spontaneous reaction and in what direction at standard conditions? The spontaneous reaction is entropy-driven to the left. The spontaneous reaction is enthalpy-driven to the right. The spontaneous reaction is entropy-driven to the right. The spontaneous reaction is enthalpy-driven to the left
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


