Question: Use the data given in the table to calculate the value of Grxn at 25C for the reaction described by the equation A+BC Grxn= If
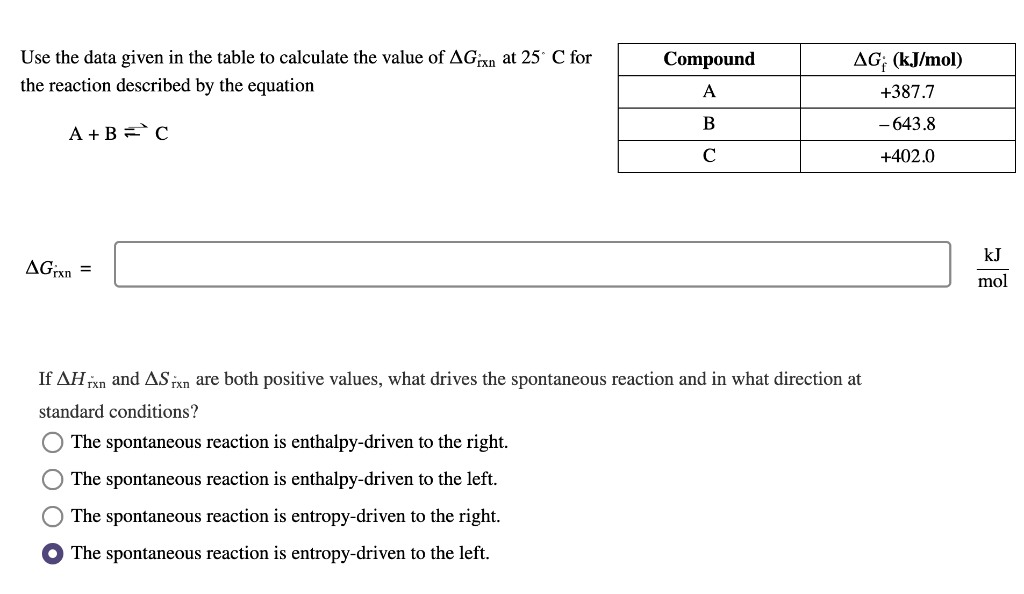
Use the data given in the table to calculate the value of Grxn at 25C for the reaction described by the equation A+BC Grxn= If Hrxn and Srxn are both positive values, what drives the spontaneous reaction and in what direction at standard conditions? The spontaneous reaction is enthalpy-driven to the right. The spontaneous reaction is enthalpy-driven to the left. The spontaneous reaction is entropy-driven to the right. The spontaneous reaction is entropy-driven to the left
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


