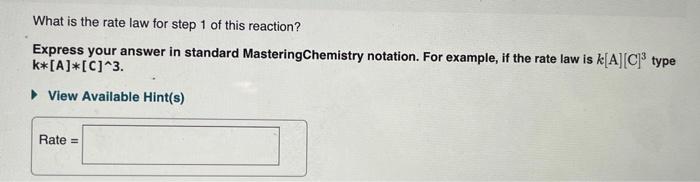
Question: please help What is the rate law for step 1 of this reaction? Express your answer in standard MasteringChemistry notation. For example, if the rate
![rate law is k[A][C]3 type k[A][C]3 View Available Hint(s) Rate = What](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f9493506760_23666f9493496a85.jpg)

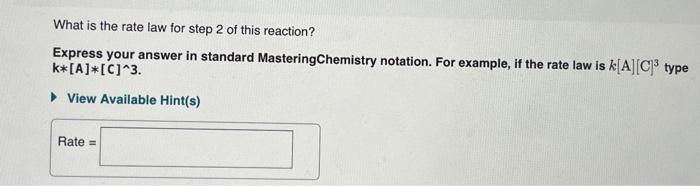
What is the rate law for step 1 of this reaction? Express your answer in standard MasteringChemistry notation. For example, if the rate law is k[A][C]3 type k[A][C]3 View Available Hint(s) Rate = What is the rate law for step 2 of this reaction? Express your answer in standard MasteringChemistry notation. For example, if the rate law is k[A][C]3 type k[A][C]3 View Available Hint(s) Rate = Order and rate law of a reaction The overall order of an elementary step directly corresponds to its molecularity. Both steps in this example are second order because they are each bimolecular. Furthermore, the rate law can be determined directly from the number of each type of molecule in an elementary step. For example, the rate law for step 1 is rate=k[NO2]2 The exponent " 2 " is used because the reaction involves two NO2 molecules. The rate law for step 2 is rate=k[NO3]1[CO]1=k[NO3][CO] because the reaction involves only one molecule of each reactant the exponents are omitted. Analyzing a new reaction Consider the following elementary steps that make up the mechanism of a certain reaction: 1. 2XY+Z 2. Y+2LM+Z Consider the second-order reaction: 2HI(g)H2(g)+I2(g) Use the simulation to find the initial concentration [HI]0 and the rate constant k for the reaction. What will be the concentration of HI after t=2.311010s([HI]t) for a reaction starting under the condition in the simulation? Express your answer in moles per liters to three significant figures. What is the rate law for step 1 of this reaction? Express your answer in standard MasteringChemistry notation. For example, if the rate law is k[A][C]3 type k[A][C]3 View Available Hint(s) Rate = What is the rate law for step 2 of this reaction? Express your answer in standard MasteringChemistry notation. For example, if the rate law is k[A][C]3 type k[A][C]3 View Available Hint(s) Rate = Order and rate law of a reaction The overall order of an elementary step directly corresponds to its molecularity. Both steps in this example are second order because they are each bimolecular. Furthermore, the rate law can be determined directly from the number of each type of molecule in an elementary step. For example, the rate law for step 1 is rate=k[NO2]2 The exponent " 2 " is used because the reaction involves two NO2 molecules. The rate law for step 2 is rate=k[NO3]1[CO]1=k[NO3][CO] because the reaction involves only one molecule of each reactant the exponents are omitted. Analyzing a new reaction Consider the following elementary steps that make up the mechanism of a certain reaction: 1. 2XY+Z 2. Y+2LM+Z Consider the second-order reaction: 2HI(g)H2(g)+I2(g) Use the simulation to find the initial concentration [HI]0 and the rate constant k for the reaction. What will be the concentration of HI after t=2.311010s([HI]t) for a reaction starting under the condition in the simulation? Express your answer in moles per liters to three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


