Question: Learning Goaly To understand how elementary stops make up a mechanism and bow the ralo law for an elementary step can be determined. Order and
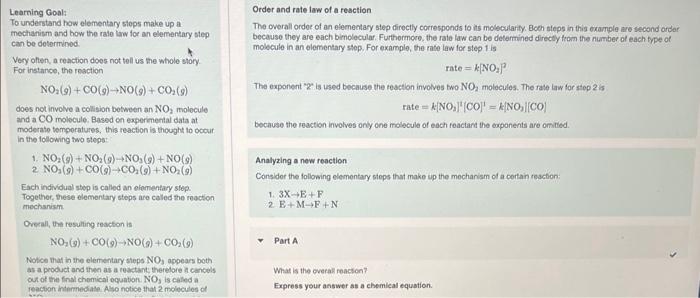

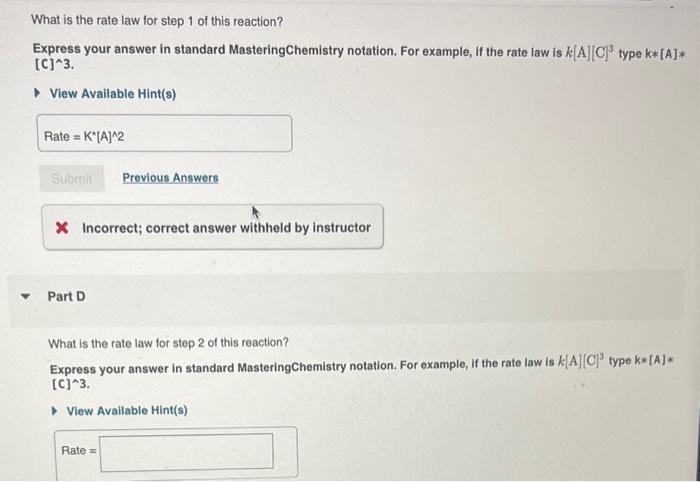
Learning Goaly To understand how elementary stops make up a mechanism and bow the ralo law for an elementary step can be determined. Order and rate law of a reaction Very often, a ceaction does not tell us the whole story. For instance, the reaction The overall ordec of an elementacy step directly corresponds to its molecularity. Bcen steps in this cxample are second order because they are each bimolecular. Furthermore, the rale law can be detemined directy thom the number of each fype of molecule in an elementary step. For example, the nite law for step 1 is NO2(g)+CO(g)NO(g)+CO2(g) The exponent " 2 " is used because the reaction involves two NO2 molocules. The rale law for step 2is does not involve a collision botween an NO2 molocule rate=k[NO3]tCO=k[NO3][CO] and a CO inolocule. Based co experimental data at moderate temporatures, this reaction is thought to oceut because the reaction involves only one molecule of each feactard the cuponents are omitied. in the following two stopat 1. NO2(g)+NO2(g)+NO2(g)+NO(g) 2. NO2(g)+CO(g)CO2(g)+NO2(g) Analyzing a new reaction Consider the following elementary stops that make up the mechanism of a cortain resction: Each individual step is called an elomentay step . Togethor, these elementary steps are caled the reaction mechanism. 1. 3XE+F Oresall, the resulting resction is NO2(9)+CO(9)NO(9)+CO2(9)PartA Notice that in the elementary sieps NO3 appears both as a product and thee as a reactint, therelore it cancels out of the fnal chemical oquation. NO3 is caled a What is the cverall reacton? reacion intermeciate. Also notice that 2 molecules of Express your answer as a chemical equation. NO2(g)+CO(g)NO(g)+CO2(g) does not involve a collision between an NO2 molecule and a CO molecule. Based on experimental data at moderate temperatures, this reaction is thought to occur in the following two steps: 1. NO2(g)+NO2(g)NO3(g)+NO(g) 2. NO3(g)+CO(g)CO2(g)+NO2(g) Each individual step is called an elementary step. Together, these elementary steps are called the reaction mechanism. Overall, the resulting reaction is NO2(g)+CO(g)NO(g)+CO2(g) Notice that in the elementary steps NO3 appears both as a product and then as a reactant; therefore it cancels out of the final chemical equation. NO3 is called a reaction intermediate. Also notice that 2 molecules of NO2 appear in the reactants of the first step and 1 molecule of NO2 appears as product of the second step, the net effect leaves only 1 molecule of NO2 as a reactant in the net equation. Molecularity is the proper term for "how the molecules collide" in a reaction. For example, step 1 is bimolecular because it involves the collision of two molecules. Step 2 is also bimolecular for the same reason. Unimolecular reactions involve only one molecule in the reactants. Though rare, collisions among three molecules can occur. Such a reaction would be called termolecular. What is the rate law for step 1 of this reaction? Express your answer in standard MasteringChemistry notation. For example, if the rate law is k[A][C]3 type k[A] [C]3. View Available Hint(s) Part D What is the rate law for step 2 of this reaction? Express your answer in standard MasteringChemistry notation. For example, if the rate law is k[A][C]3 type k[A] [C]3. View Available Hint(s) Rate =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


