Question: please help When silicon is doped with phosphorus, a group 5A element with five valence electrons, its conductivity The phosphorus atoms are incorporated into the
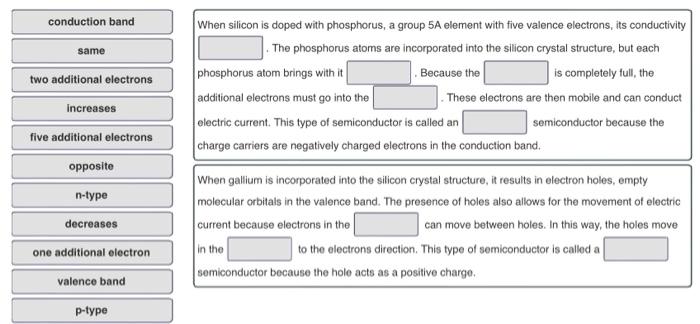
When silicon is doped with phosphorus, a group 5A element with five valence electrons, its conductivity The phosphorus atoms are incorporated into the silicon crystal structure, but each phosphorus atom brings with it Because the is completely full, the additional electrons must go into the These electrons are then mobile and can conduct electric current. This type of semiconductor is called an semiconductor because the charge carriers are negatively charged electrons in the conduction band. When gallium is incorporated into the silicon crystal structure, it results in electron holes, empty molecular crbitals in the valence band. The presence of holes also allows for the movement of electric current because electrons in the can move between holes. In this way, the holes move in the to the electrons direction. This type of semiconductor is called a semiconductor because the hole acts as a positive charge
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


