Question: please help When the following skeletal equation is balanced under acidic conditions, what are the coefficients of the species shown? HNO3+NiNO+Ni2+ Water appears in the
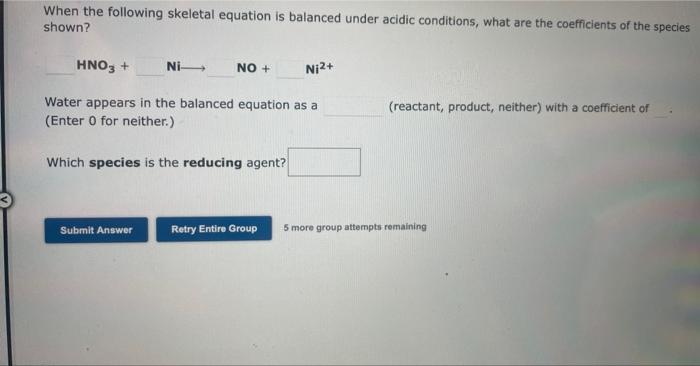
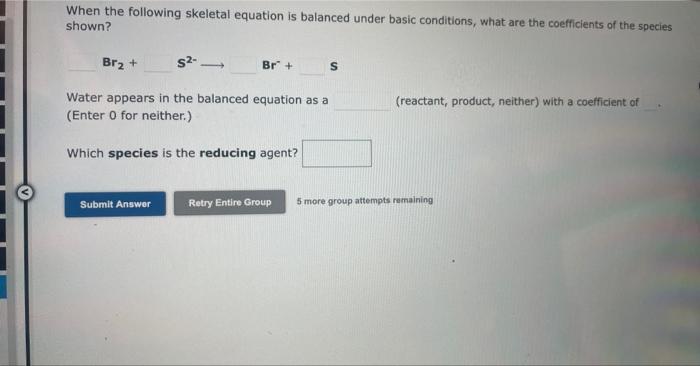
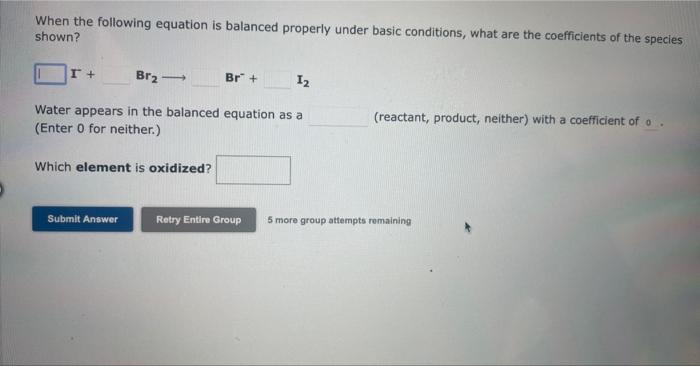
When the following skeletal equation is balanced under acidic conditions, what are the coefficients of the species shown? HNO3+NiNO+Ni2+ Water appears in the balanced equation as a (reactant, product, neither) with a coefficient of (Enter 0 for neither.) Which species is the reducing agent? 5 more group attempts remaining When the following skeletal equation is balanced under basic conditions, what are the coefficients of the species shown? Br2+S2Br+S Water appears in the balanced equation as a (Enter 0 for neither.) (reactant, product, neither) with a coefficient of Which species is the reducing agent? 5 more group attempts remaining When the following equation is balanced properly under basic conditions, what are the coefficients of the species shown? I+Br2Br+I2 Water appears in the balanced equation as a (Enter 0 for neither.) (reactant, product, neither) with a coefficient of 0 . Which element is oxidized? 5 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


