Question: please help with #2 or #3 plsss 2. The vinegar bottle advertises that it is 5% acidity. Is this a valid advertisement based on

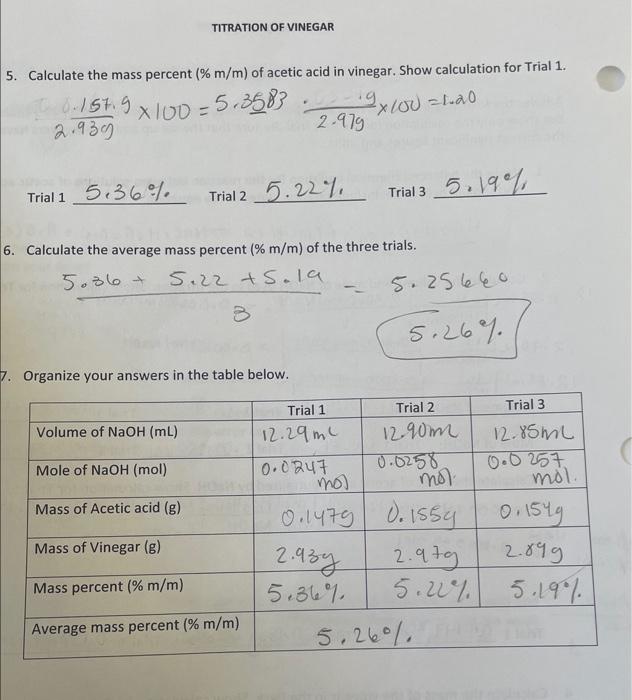
2. The vinegar bottle advertises that it is " 5% acidity". Is this a valid advertisement based on your data? 3. One of the common errors in this experiment is overshooting the equivalence point (i.e. adding too much NaOH ). Doe this error cause an increase or decrease in the calculated mass percent? Explain. 4. Write the total ionic and net ionic equations for the reaction between acetic acid and NaOH. Show physical states of all reactants and products. (Note: acetic acid is a weak acid, write it in the molecular form - do not ionize). 5. Calculate the mass percent (%m/m) of acetic acid in vinegar. Show calculation for Trial 1. Trial 1 S.36\% Trial 5.22% Trial 3.19% 6. Calculate the average mass percent (%m/m) of the three trials. 35.36+5.22+5.19=5.26%5.25660 Organize your answers in the table below
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


