Question: please help with advanced reaction engineering!! Ine reaction: A+2B3C has elementary kinetics and is done in a gas phase batch reactor at constant volume. There
please help with advanced reaction engineering!!
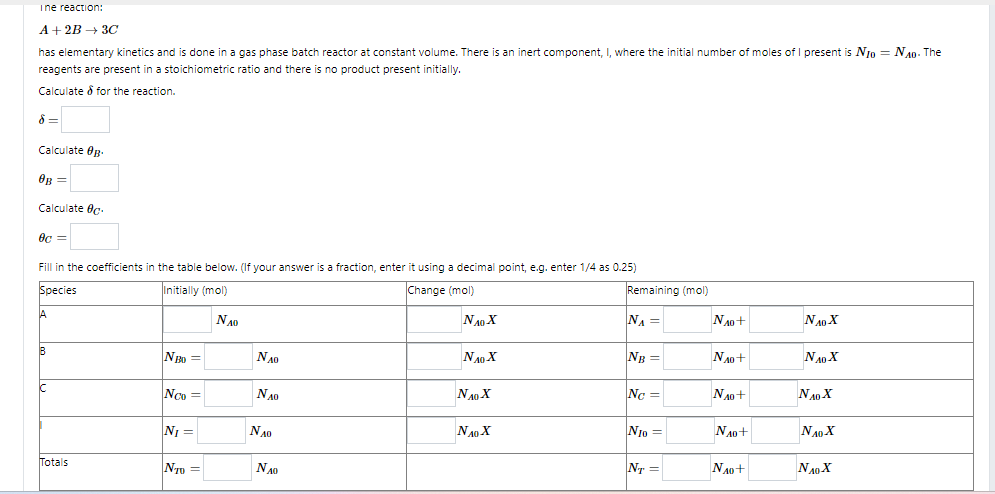
Ine reaction: A+2B3C has elementary kinetics and is done in a gas phase batch reactor at constant volume. There is an inert component, I, where the initial number of moles of I present is NI0=NA0. The reagents are present in a stoichiometric ratio and there is no product present initially. Calculate for the reaction. = Calculate B. B= Calculate C. c= Ine reaction: A+2B3C has elementary kinetics and is done in a gas phase batch reactor at constant volume. There is an inert component, I, where the initial number of moles of I present is NI0=NA0. The reagents are present in a stoichiometric ratio and there is no product present initially. Calculate for the reaction. = Calculate B. B= Calculate C. c=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


