Question: please help with both question A 312g sample of a metal is heated to 322.243C and plunged into 200g of water at a temperature of
please help with both question 
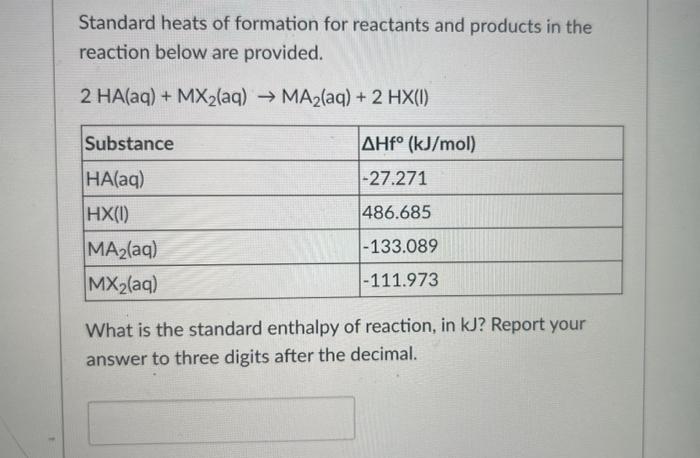


A 312g sample of a metal is heated to 322.243C and plunged into 200g of water at a temperature of 38.575C. The final temperature of the water is 70.978C. Assuming water has a specific heat capacity of 4.184J/gC, what is the specific heat capacity of the metal sample, in J/gC )? Assume no heat loss to the surroundings. Report your response to 3 digits after the decimal. Standard heats of formation for reactants and products in the reaction below are provided. 2HA(aq)+MX2(aq)MA2(aq)+2HX(I) What is the standard enthalpy of reaction, in kJ? Report your answer to three digits after the decimal
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


