Question: please help with equilibrium constants and activation energy using the chart above! *Remember to use concentrations AFTER mixing * Divide [Na2S2O3] by the time Order
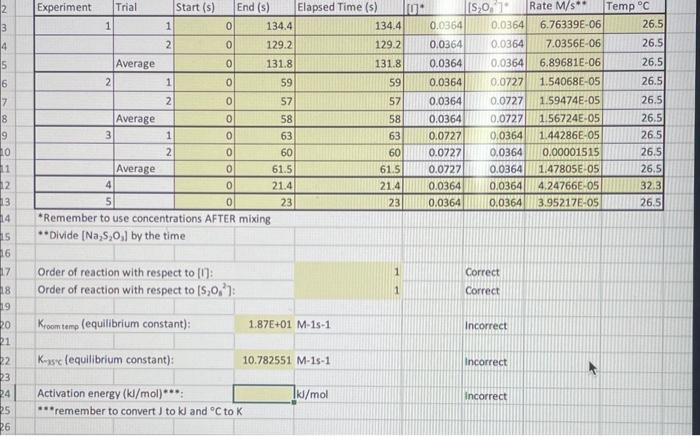
*Remember to use concentrations AFTER mixing * Divide [Na2S2O3] by the time Order of reaction with respect to [1]: Order of reaction with respect to [S2O82] : 11CorrectCorrect Kroomtemp (equilibrium constant): 1.87E+01M1s1 Incorrect K-asc (equilibrium constant): 10.782551M1s1 incorrect Activation energy (kJ/mol) : 1ks/mol incorrect "remember to convert J to KJ and C to K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


