Question: please help with part a and b, important Calculate the vapour presseire of a solution containing 23.8g of 1.3.proparediel (C3HsO2) in 123mL of watee at
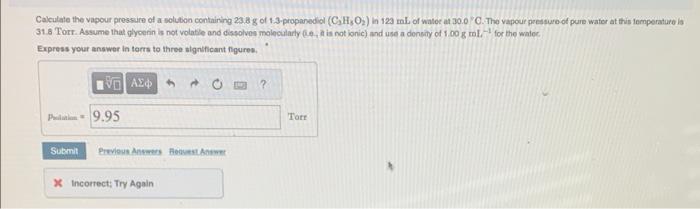
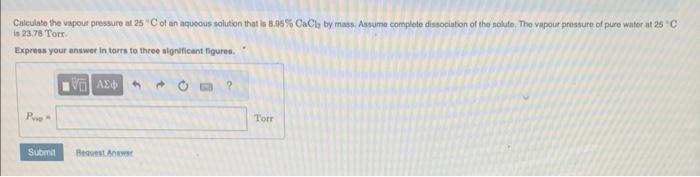
Calculate the vapour presseire of a solution containing 23.8g of 1.3.proparediel (C3HsO2) in 123mL of watee at 30.0 ' C. The vapour pressure of pure water at this iemperature is 31.8 Torr. Assume that plyoenin is not volatile and dissolvos molecularly ( 1 e., it is not ionic) and use a density of 1.00g ml. 1 for the walec, Express your answer in torrs to three signsficant figures, Calculate the vapout pressure at 25C of an aqueous solution that is 8.35%CaCl by mass. Assume complete dissociabon of the solule, The vapour pressure of pure water at 25C. is 23.78 Torr. Exproas your answer in torrs to three algnificant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


