Question: Please help with part A, B, and C Part A Determine which of the foliowing reactions would undergo deprotonation based on the strength of the
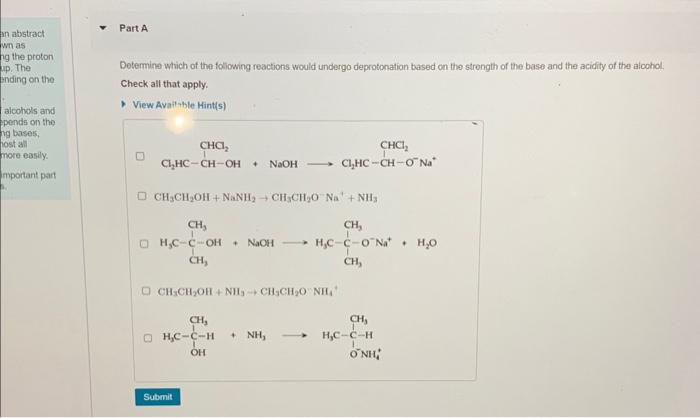
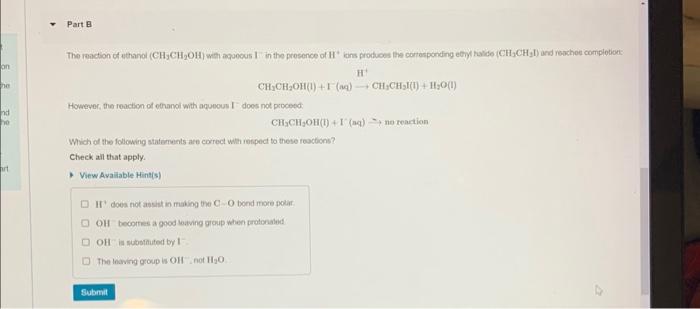
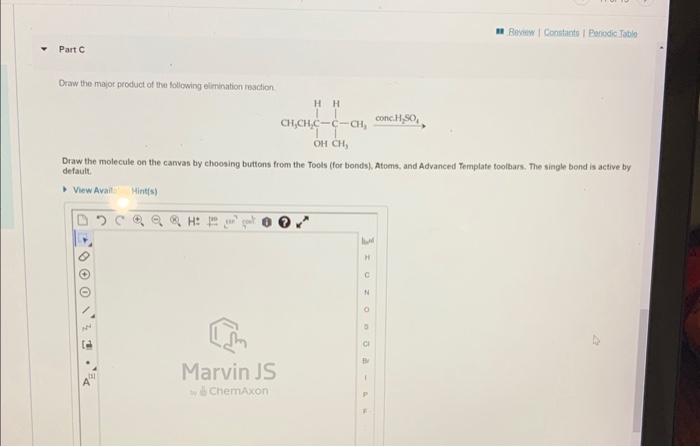
Part A Determine which of the foliowing reactions would undergo deprotonation based on the strength of the base and the acidity of the alcohol; Check all that apply. View Available Hint(s) CH3CH2OH+NaNH2CH3CH2ONa++NH3 + NaOH H2O CH3CH2OH+NH3+CH3CH2ONH44 However, the reacion of echanol with aqueous I does not proceed: CH7CH9OH(I)+I(aq)-noteartion Which of the following staternents are correct with renpect to these roachions? Check ail that apply. View Avaliable Hint(s) II doea not absist in mabing the C. O bond more polar OIf boontres a good lowing group when protonalud OII is subbinuted by 1 The Inaving group is OHI, not II2O Draw the major product of tha following elrnination reacion Draw the molecule on the canvas by choosing buttons from the Tools (for bonds). Atoms, and Advanced Template toolbars. The single bond is active by. default. Yew Ayail. Mints(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


