Question: please help with question 2 specifically Assuming a mixture of n-pentane (1) and n-heptane (2) is ideal, prepare vapour-liquid equilibrium diagrams (xy, and Pxy) for
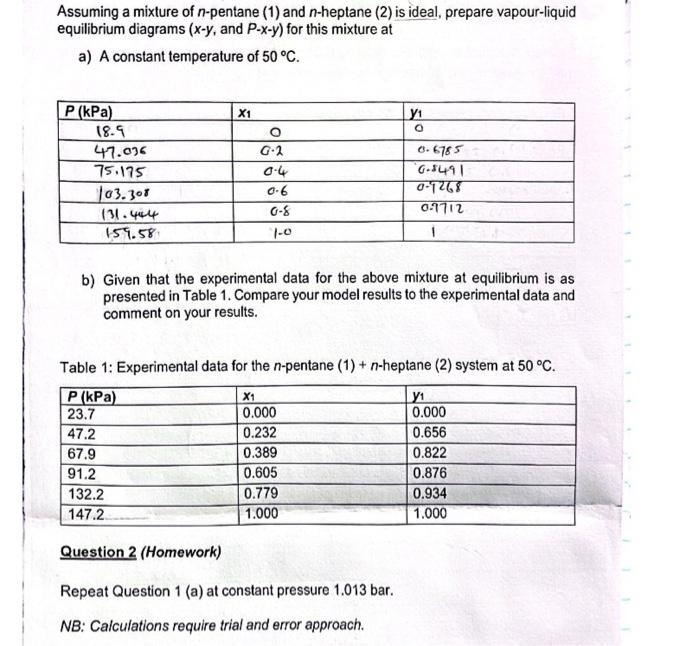
Assuming a mixture of n-pentane (1) and n-heptane (2) is ideal, prepare vapour-liquid equilibrium diagrams (xy, and Pxy) for this mixture at a) A constant temperature of 50C. b) Given that the experimental data for the above mixture at equilibrium is as presented in Table 1. Compare your model results to the experimental data and comment on your results. Table 1: Experimental data for the n-pentane (1) +n-heptane (2) system at 50C. Question 2 (Homework) Repeat Question 1 (a) at constant pressure 1.013 bar. NB: Calculations require trial and error approach
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


