Question: please help with question 3 and 4 only. 1. Two of the liquids, n-pentane and 1-butanol, had nearly the same molecular weights, but significantly different
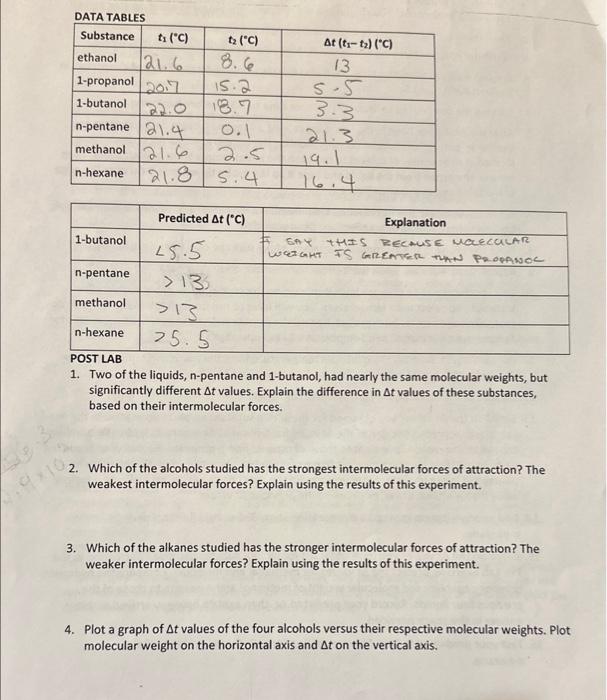
1. Two of the liquids, n-pentane and 1-butanol, had nearly the same molecular weights, but significantly different t values. Explain the difference in t values of these substances, based on their intermolecular forces. 2. Which of the alcohols studied has the strongest intermolecular forces of attraction? The weakest intermolecular forces? Explain using the results of this experiment. 3. Which of the alkanes studied has the stronger intermolecular forces of attraction? The weaker intermolecular forces? Explain using the results of this experiment. 4. Plot a graph of t values of the four alcohols versus their respective molecular weights. Plot molecular weight on the horizontal axis and t on the vertical axis
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


