Question: please help with questions 1 and 2 DATA TARIEE 1. Two of the liquids, n-pentane and 1-butanol, had nearly the same molecular weights, but significantly
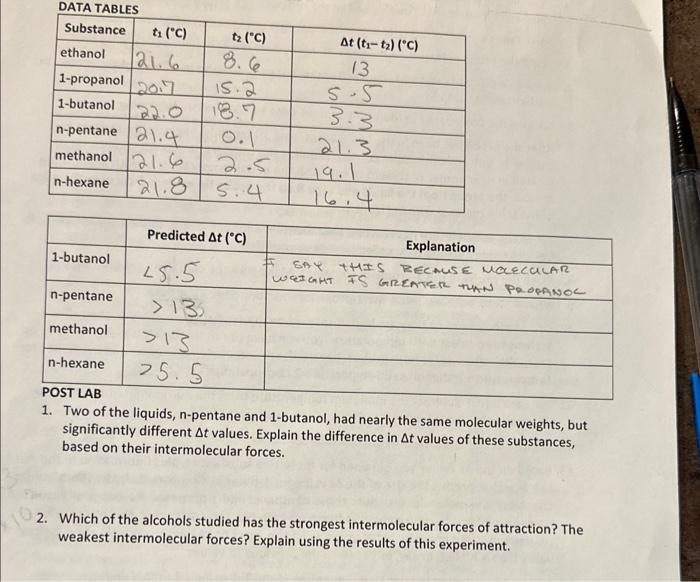
DATA TARIEE 1. Two of the liquids, n-pentane and 1-butanol, had nearly the same molecular weights, but significantly different t values. Explain the difference in t values of these substances, based on their intermolecular forces. 2. Which of the alcohols studied has the strongest intermolecular forces of attraction? The weakest intermolecular forces? Explain using the results of this experiment. DATA TARIEE 1. Two of the liquids, n-pentane and 1-butanol, had nearly the same molecular weights, but significantly different t values. Explain the difference in t values of these substances, based on their intermolecular forces. 2. Which of the alcohols studied has the strongest intermolecular forces of attraction? The weakest intermolecular forces? Explain using the results of this experiment
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


