Question: please help with question e and f e. Calculate the specific rate constant for this reaction. Specify units. f. Calculate the concentration of X in

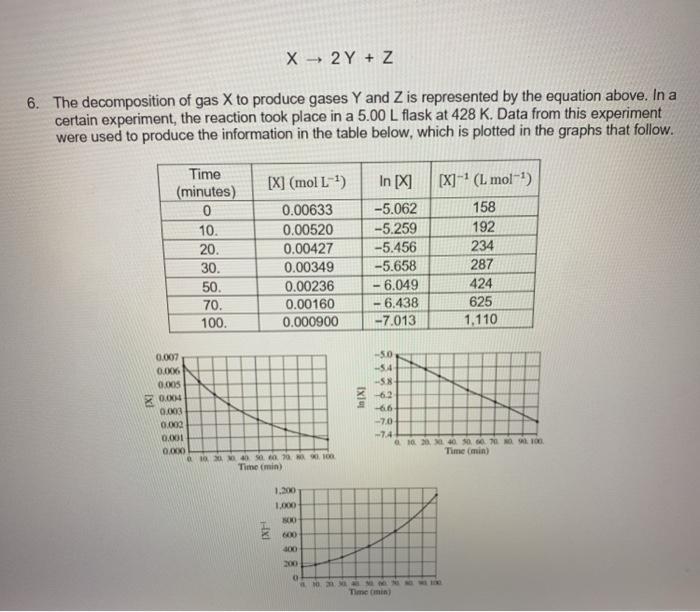
e. Calculate the specific rate constant for this reaction. Specify units. f. Calculate the concentration of X in the flask after a total of 120. minutes of reaction. X 2 Y + Z 6. The decomposition of gas X to produce gases Y and Z is represented by the equation above. In a certain experiment, the reaction took place in a 5.00 L flask at 428 K. Data from this experiment were used to produce the information in the table below, which is plotted in the graphs that follow. [X] (mol L-) Time (minutes) 0 10. 20. 30. 50. 70. 100. 0.00633 0.00520 0.00427 0.00349 0.00236 0.00160 0.000900 In [X] -5.062 -5.259 -5.456 -5.658 - 6.049 6.438 -7.013 [X]^? (L mol-) 158 192 234 287 424 625 1,110 In XI 0.007 0.00 0.005 0.004 0.003 0.000 0.001 OOO 140 secon Time (min) -50 -54 -58 -6.2 -6.6 - 7.0 -74 100400000 Time min) 1.300 1.000 SOO INT 200 Time mini
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


