Question: please help with section b only Thermodynamic data that may be useful in solving this problem are tabulated below. Values are for 25C and 1
please help with section b only
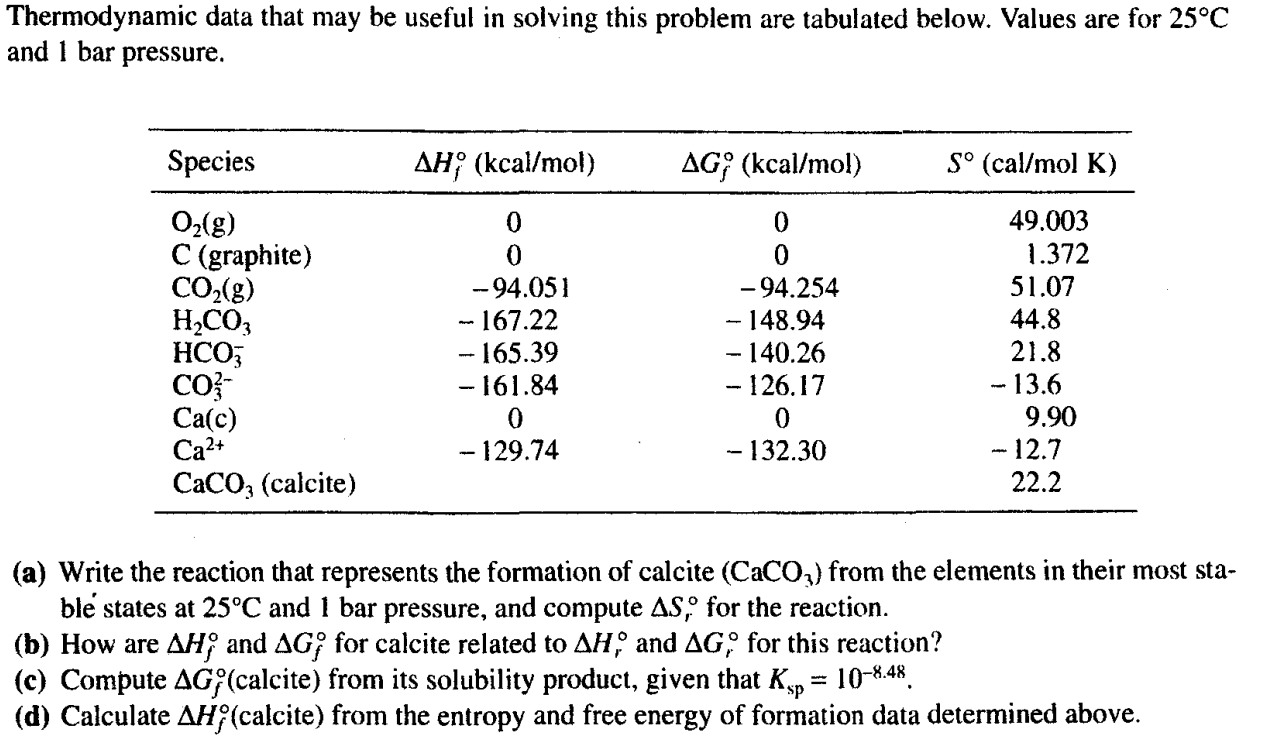
Thermodynamic data that may be useful in solving this problem are tabulated below. Values are for 25C and 1 bar pressure. (a) Write the reaction that represents the formation of calcite (CaCO3) from the elements in their most stabl states at 25C and 1 bar pressure, and compute Sr for the reaction. (b) How are Hf and Gf for calcite related to Hr and Gr for this reaction? (c) Compute Gf (calcite) from its solubility product, given that Ksp=108.48. (d) Calculate Hf (calcite) from the entropy and free energy of formation data determined above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


